Compound aspirin tading kind medicinal preparation and its application
A technology of aspirin and medicine, which is applied in compound aspirin statin drug preparations and its application field, and can solve problems such as stimulation reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: prepare common tablet
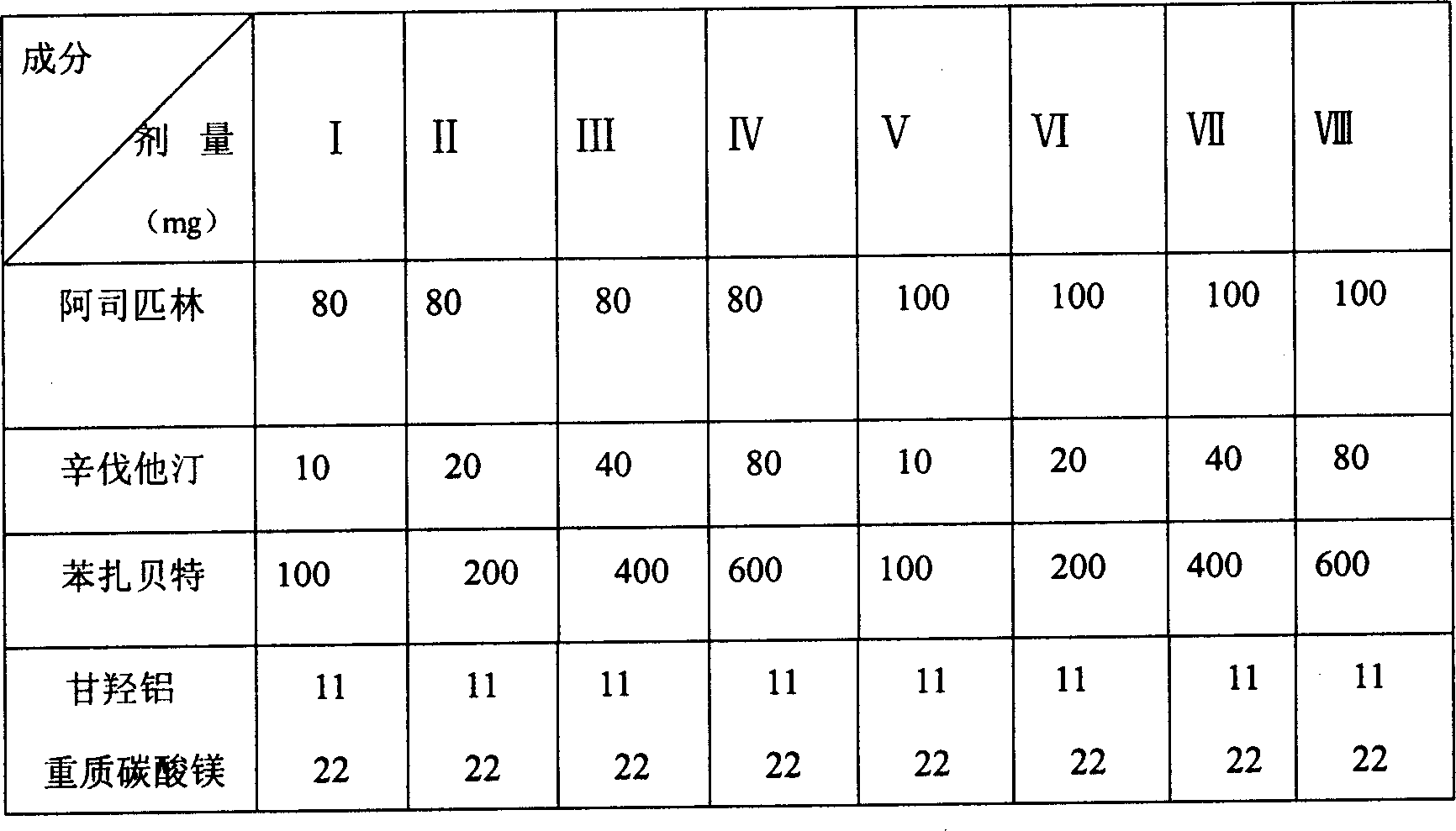
[0045] Element
[0046] Preparation method: the drug and auxiliary materials are passed through 80 mesh sieves, aspirin, lovastatin, clofibrate, aluminum glycolate, heavy magnesium carbonate, microcrystalline cellulose, cross-linked carboxymethyl cellulose (2 / 3), Mix well, use 5% povidone 95% ethanol solution to make soft material, sieve, dry, granulate, add remaining 1 / 3 cross-linked carboxymethyl cellulose, magnesium stearate, micropowder silica gel, mix well, directly Tablets, ready to use.
Embodiment 2
[0047] Embodiment 2: Preparation of enteric-coated tablets
[0048] According to Example 1, 1000 ordinary compound aspirin tablets were prepared and coated with an enteric coating material.
[0049] prescription:
[0050] 1000 tablets
[0051] Hydroxypropyl Methyl Cellulose Phthalate 21g Coating Polymer
[0052] Ethylcellulose 3.6g Film coating aid
[0053] Ethanol 130ml organic solvent
[0054] Acetone 130ml organic solvent
[0055] Coating with a coating pan
[0056] Operating conditions:
[0057] Coating pan speed 6~7r / min
[0058] Spray Type Airless Spray
[0059] Nozzle size 0.4mm nozzle (60° angle)
[0060] Spray speed 100ml / min
[0061] Liquid pressure 52~80kg / cm 2
[0062] Exhaust velocity 140~160m 3 / min
[0063] Intake air velocity 130~150m 3 / min; 20℃
[0064] 40%~60% relative humidity
[0065] Coating time 45min
[0066] Tablet weight increased by about 5% after coating.
Embodiment 3
[0068] Element
[0069] Preparation method: pass through 80-mesh sieve respectively for the drug and auxiliary materials, mix aspirin, fluvastatin, gemfibrozil, aluminum glycolate, heavy magnesium carbonate, microcrystalline cellulose, and cross-linked carboxymethyl cellulose evenly, and use 5% povidone 75% ethanol solution to make soft materials, sieve, dry, granulate, add talcum powder, mix well, put into capsule shell.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com