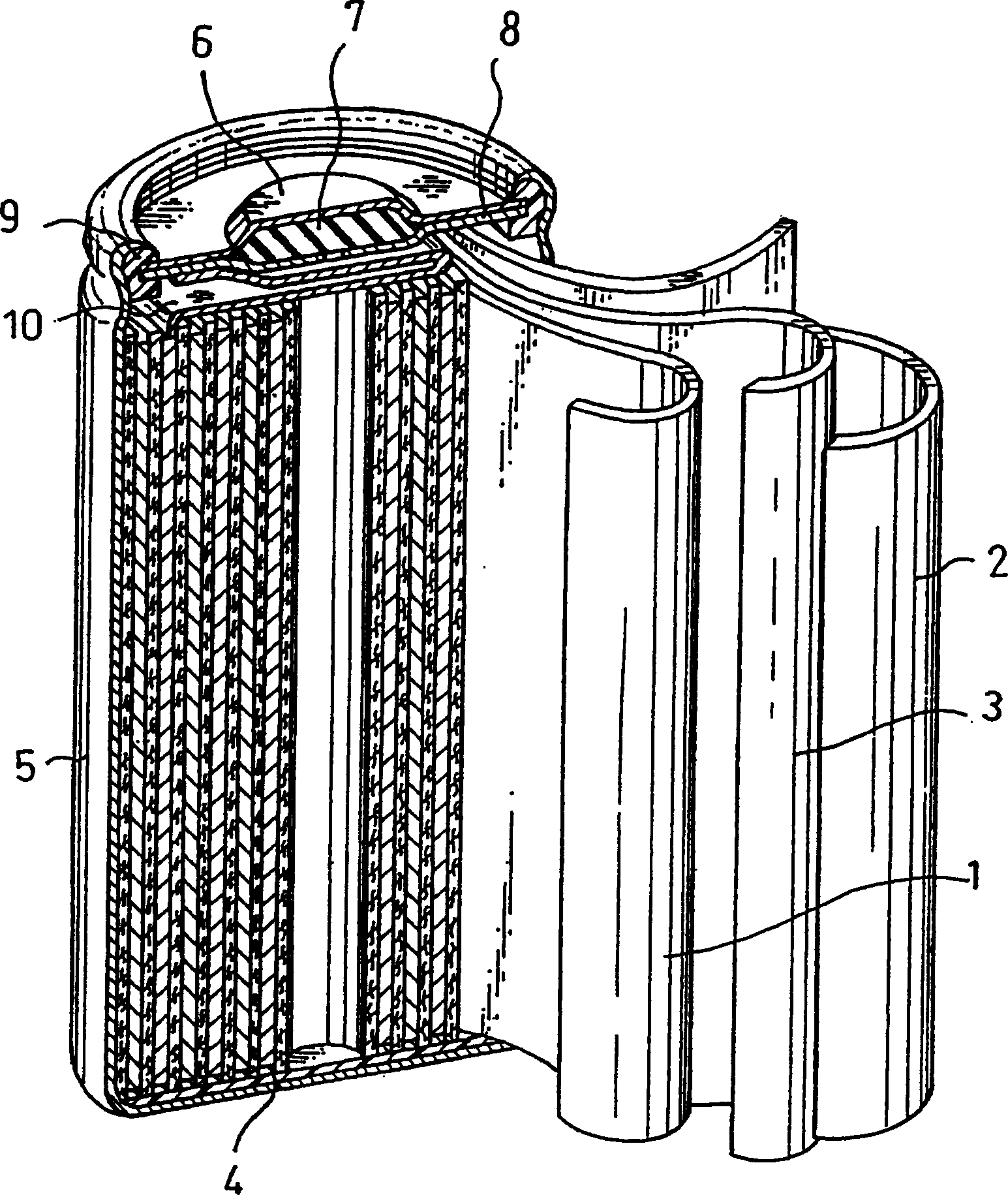
Alkaline storage battery and method for producing the same
A manufacturing method and storage battery technology, which are applied in the manufacturing of alkaline storage batteries, alkaline storage batteries, electrodes of alkaline storage batteries, etc., can solve problems such as inability to eliminate, and achieve the effects of suppressing manufacturing costs, low cost, and less self-discharge
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] (i) Preparation of positive electrode
[0083] Nickel carbonyl powder (#255 manufactured by INCO Corporation) was dispersed in an aqueous solution containing 1% by weight of methylcellulose to prepare a slurry having a solid content of 35% by weight. Gained slurry is coated on the surface of metal substrate, so that the weight density of the nickel powder after sintering is 50g / m 2 . The metal substrate is made by electroplating method, with a thickness of 20μm and a weight density of 170g / m 2 nickel foil.
[0084] Next, the nickel foil with the slurry was dried to evaporate water, and then sintered at 950° C. for 15 minutes in a hydrogen-reducing atmosphere containing water vapor. As a result, organic substances contained in the slurry were removed, and a sintered product of nickel foil and nickel powder (hereinafter referred to as nickel sintered substrate) was obtained.
[0085] The resulting nickel sintered substrate was cut into a matrix to make cuts (slits) of...
Embodiment 2
[0115] (i) Production of batteries
[0116] When making the separator layer, use the average particle size (median particle size D 50 ) 0.2μm carbon fluoride (CF 1.0 ) instead of polytetrafluoroethylene, a sealed battery with a nominal capacity of 6000 mAh in the initial state was produced in the same manner as in Example 1.
[0117] The batteries using zinc oxide, iron oxide, magnesium oxide, manganese oxide, aluminum oxide, chromium oxide, vanadium oxide, titanium oxide and cobalt oxide as oxygen-containing metal compounds are respectively referred to as example batteries C-1, C-2, C-3, C-4, C-5, C-6, C-7, C-8 and C-9.
[0118] (ii) Evaluation of batteries
[0119] Next, the same evaluation as in Example 1 was performed using batteries C-1 to C-9. The results are shown in Table 2.
[0120]
Embodiment 3
[0122] (i) Fabrication of separator layer (polymer sheet)
[0123] Add 125 g of potassium hydroxide aqueous solution (alkaline aqueous solution) with a specific gravity of 1.25 g / ml to 10 g of cross-linked potassium polyacrylate (water-absorbent polymer), 0.2 g of carboxymethyl cellulose (binder), 6.75 g of average Particle size (median particle size D 50 ) 0.1μm polytetrafluoroethylene powder (hydrophobic agent) and 0.5g average particle size (median particle size D 50 ) 1 μm predetermined oxide (oxygen-containing metal compound) and mixed to make it gel.
[0124] The obtained gel was impregnated into the polypropylene nonwoven fabric used in Comparative Example 1 and dried. Thus, a polymer sheet having a thickness of 80 µm was obtained.
[0125] The air permeability of the obtained polymer sheet is about 18ml / cm under the condition of a pressure difference of 124Pa 2 ·s.
[0126] (ii) Production of batteries
[0127] A sealed battery having a nominal capacity of 6000 mAh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com