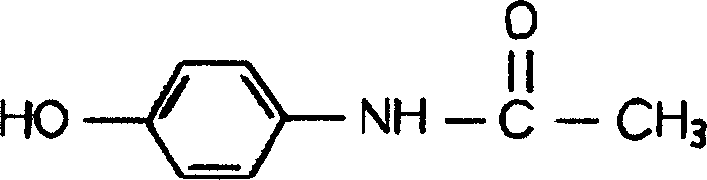
Oral disintegration tablets contg. p-acetaminophenol, and prepn. method therefor
A technology of acetaminophen and orally disintegrating tablets, which is applied in medical formulas, medical preparations containing active ingredients, pill delivery, etc., can solve problems such as difficulty in swallowing patients, inconvenient taking, and high hardness of tablets, and achieve Improve bioavailability, easy to take, and accelerate absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: Preparation of acetaminophen orally disintegrating tablets
[0046] formula:
[0047] Component Weight Weight %
[0048] Acetaminophen 100g 24%
[0049] Mannitol 160g 38.4%
[0050] Aspartame 1.2g 0.28%
[0051] Sodium bicarbonate 5g 1.2%
[0052] Citric Acid 5.7g 1.37%
[0053] Microcrystalline Cellulose 120g 28.8%
[0054] Polyvinylpyrrolidone (PVPP) 20g 4.8%
[0055]Flavor 0.8g 0.2%
[0056] Talc 4g 0.96%
[0057] Total weight 416.7 grams
[0058] A total of 1000 pieces were made
[0059] Preparation Process:
[0060] Weigh acetaminophen, mannitol, and aspartame according to the prescription amount, stir and mix well, use 50% ethanol aqueous solution (V / V) to make a soft material, pass through a 32-mesh sieve to granulate, dry at 50°C, press Prescription weighs additional auxiliary materials sodium bicarbonate, citric acid, microcrystalline cellulose, polyvinylpyrrolidone (PVPP), essence, talcum powder, mixes evenly, and compresses tablets. ...
Embodiment 2
[0061] Example 2: Preparation of acetaminophen orally disintegrating tablets
[0062] formula:
[0063] Component Weight Weight %
[0064] Acetaminophen 100g 20%
[0065] Erythrose 240g 47.9%
[0066] Cyclamate 7.2g 1.44%
[0067] Sodium bicarbonate 5 g 1%
[0068] Citric Acid 5.7g 1.1%
[0069] Microcrystalline Cellulose 98g 19.57%
[0070] Low-substituted hydroxypropyl cellulose (L-HPC) 40g 7.98%
[0071] Flavor 0.8g 0.16%
[0072] Talc 4g 0.8%
[0073] Total weight 500.7 grams
[0074] A total of 1000 pieces were made
[0075] Preparation Process:
[0076] Weigh acetaminophen, erythrose, cyclamate, and microcrystalline cellulose (half the amount) according to the prescription, stir and mix well, use 50% ethanol aqueous solution (V / V) to make a soft material, and pass through a 32-mesh sieve Granules, dried, weighed according to the prescription plus excipients sodium bicarbonate, citric acid, microcrystalline cellulose, low-substituted hydroxypropyl cellulose (L...
Embodiment 3
[0077] Embodiment 3: Preparation of acetaminophen orally disintegrating tablets
[0078] formula:
[0079] Component Weight Weight %
[0080] Acetaminophen 100g 24.8%
[0081] Erythrose 140g 34.76%
[0082] Cyclamate 7.2g 1.79%
[0083] Sodium bicarbonate 5g 1.24%
[0084] Citric Acid 5.7g 1.42%
[0085] Microcrystalline Cellulose 120g 29.8%
[0086] Low Substituted Hydroxypropyl Cellulose (L-HPC) 20g 5%
[0087] Flavor 0.8g 0.2%
[0088] Talc 4g 0.99%
[0089] Total weight 402.7 grams
[0090] A total of 1000 pieces were made
[0091] Preparation Process:
[0092] Weigh acetaminophen, erythrose, and cyclamate according to the prescription amount, stir and mix evenly, use 50% ethanol aqueous solution (V / V) to make a soft material, pass through a 32-mesh sieve to granulate, dry, and weigh according to the prescription Add excipients sodium bicarbonate, citric acid, microcrystalline cellulose, low-substituted hydroxypropyl cellulose (L-HPC), essence, talcum powder, m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com