Method of preparing adipinic acid using bionic catalytic oxggen to oxidize cyclohexane
A technology of oxygen oxidation and biomimetic catalysis, applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylate, etc., can solve the problem of high energy consumption, and achieve the effect of simple reaction operation, easy operation and good catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
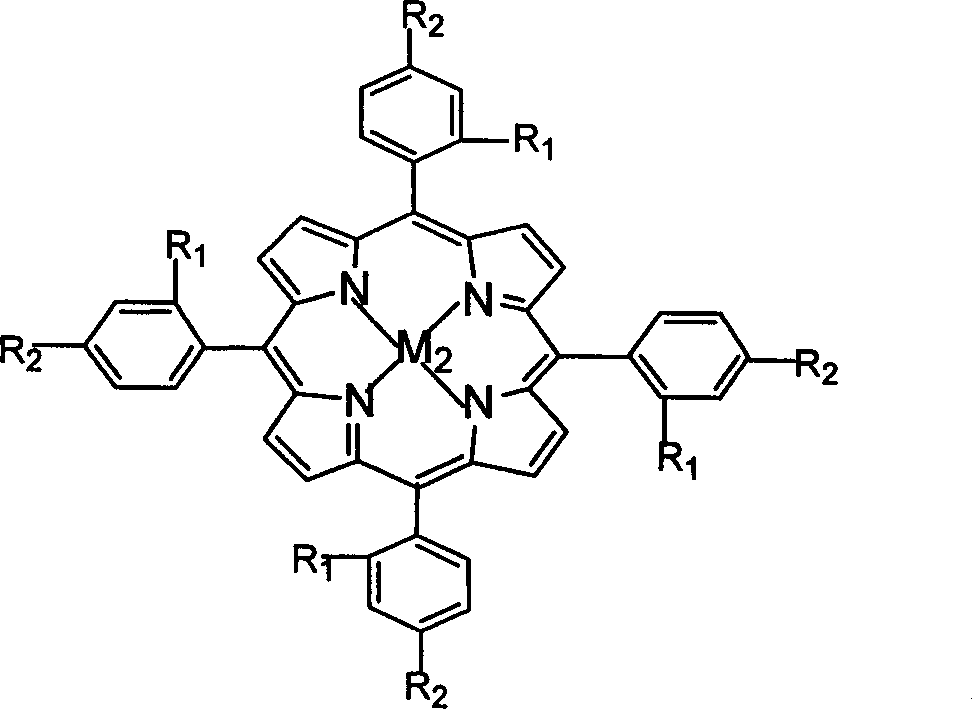
[0020] Take by weighing 1mg four-(o-chlorophenyl) iron porphyrin (i.e. R in the general formula (II) 1 = Cl, R 2 = H, M 2 =Fe), take 15mL (11.7g) of cyclohexane, put it into a 200mL autoclave, feed oxygen, the pressure is 2.5MPa, the temperature of the oil bath is controlled at 140°C, and react for 8h. The reaction mixture was filtered under reduced pressure to achieve solid-liquid separation, and the liquid phase was placed in a refrigerator to cool for a certain period of time, and a solid substance was precipitated in the solution, which was filtered and combined with the aforementioned solid, recrystallized, dried and weighed. The yield of adipic acid obtained by high-pressure liquid chromatography analysis and detection is 18.2%, and the purity of the purified product is 99.0%.
Embodiment 2
[0022] Take by weighing 2mg four-(o-chlorophenyl) cobalt porphyrin (being R in general formula (II) 1 = Cl, R 2 = H, M 2 =Co), measure 15mL of cyclohexane, put it into a 200mL autoclave, feed oxygen, the pressure is 0.5MPa, the temperature of the oil bath is controlled at 150°C, and react for 12h. The processing steps are the same as in Example 1. The obtained product was analyzed and detected by high-pressure liquid chromatography, and the yield of adipic acid was 6.9%, and the purity of the purified product was 99.4%.
Embodiment 3
[0024] Weigh 1mg of four-(o-chlorophenyl) manganese porphyrin (i.e. R in the general formula (II) 1 = Cl, R 2 = H, M 2 =Mn), take 15mL of cyclohexane, put it into a 200mL autoclave, feed oxygen, the pressure is 1.0MPa, the temperature of the oil bath is controlled at 145°C, and react for 10h. The processing steps are the same as in Example 1. The obtained product was analyzed and detected by high-pressure liquid chromatography, and the yield of adipic acid was 8.6%, and the purity of the purified product was 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com