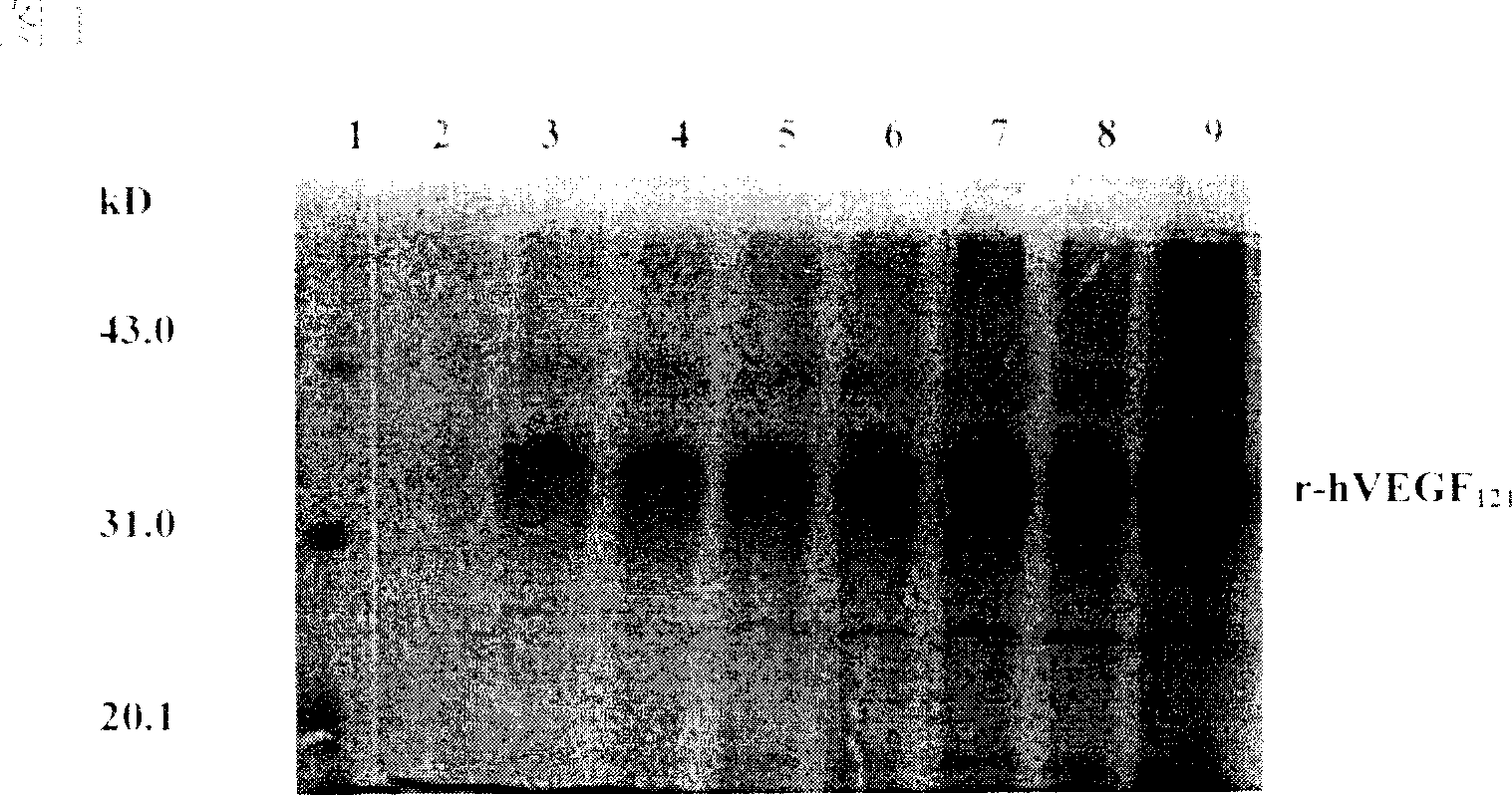Method for preparing growth factor of recombining endothelial cells of human blood vessel
A vascular endothelium and growth factor technology is applied in the field of preparation of recombinant human vascular endothelial growth factor 121, which can solve the problems of low expression of insect cells and mammalian cells, difficulty in renaturation during expression in Escherichia coli, and complicated purification. The effect of high-efficiency promoting vascular endothelial cell proliferation, high-efficiency promoting vascular permeability, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Embodiment 1 design, prepare r-hVEGF 121
[0013] (1) Clone VEGF 121 cDNA gene, construction of expression plasmid p9KVEGF 121 .
[0014] According to hVEGF 121 The amino acid sequence of Pichia pastoris was used to design primer sequences according to the preferred codons of Pichia pastoris. The gene was amplified by RT-PCR and recombined with pUC19, transformed into Escherichia coli JM109, extracted the plasmid, identified by enzymatic digestion with corresponding restriction endonucleases, obtained characteristic fragments, and confirmed the positive clones. Nucleotide sequence analysis confirmed that the gene sequence was correct. VEGF 121 The cDNA gene was cut out and recombined with the expression vector pPIC9K of Pichia pastoris to construct the eukaryotic expression plasmid p9KVEGF 121 . Transform Escherichia coli JM109, extract the plasmid and perform corresponding restriction endonuclease analysis to obtain characteristic fragments and confirm the posi...
Embodiment 2
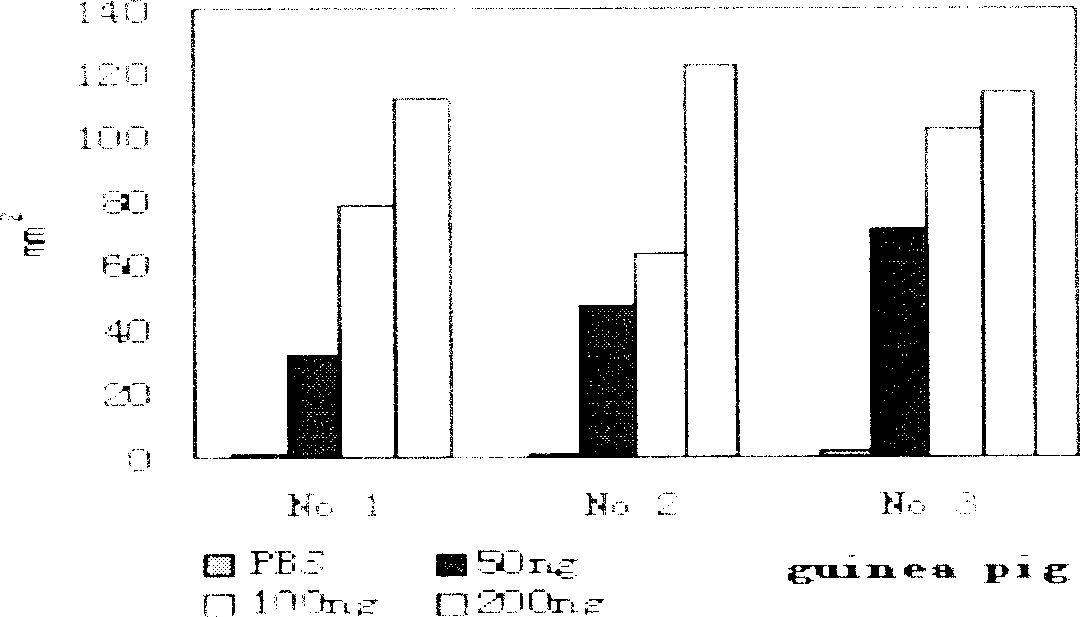
[0033] r-hVEGF 121 Biological identification (1) Bovine capillary endothelial (BCE) cells in vitro
[0034] Proliferation assay
[0035] DMEM medium containing 10% calf serum at 37°C, 5% CO 2 The BCE cell strain was subcultured under conditions, the cells were rinsed with PBS, digested with trypsin, and the cell suspension was made into a cell suspension of 1.25×10 4 / ml, inoculated into a 48-well plate and continued to culture for 24 hours, aspirate and discard the culture medium, add 0.4ml culture medium and 10μl different concentrations of r-hVEGF to the measurement group 121 For the sample, add 0.4ml culture medium and 10μl PBS to the control group, incubate at room temperature for 20 minutes, then add culture medium to make the volume of culture medium in each well 0.8ml, repeat the above steps after 48 hours, count the white blood cells under a low-magnification microscope after 72 hours BCE cells, statistical analysis of variance results showed that r-hVEGF 121 Can ...
Embodiment 3
[0041] r-hVEGF 121 Use in ischemic diseases
[0042] (1) Promote the formation of collateral vessels in the ischemic hindlimb of rabbits
[0043] Adult, male New Zealand rabbits were ligated at the distal end of the external iliac artery, and the common femoral artery was removed to create a model of occlusive vascular disease in the lower extremities. After 10 days, the treatment group was given r-hVEGF intravenously 121 1.0-3.0 mg / kg, the negative control group was given normal saline, and angiography was performed 10 days after administration. Angiography results showed that r-hVEGF 121 The new blood vessels in the treatment group were significantly more than those in the control group, and the establishment of collateral circulation was better than that in the control group.
[0044] (2) Promote the formation of collateral vessels in the ischemic myocardium of minipigs
[0045] After thoracotomy in minipigs, the Ameroid constrictor was placed on the proximal circumflex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com