Coculine osmosis pump type release-controlled preparation and preparation process thereof
A controlled-release preparation, the technology of sinomenine, is applied in the directions of anti-inflammatory agents, pharmaceutical formulations, non-central analgesics, etc., which can solve the problems of difficulty in controlling the release rate, difficulty in industrialized mass production, and large differences in product batches, etc. , to achieve stable release, reduce peak-to-valley phenomenon, and reduce the number of doses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Tablet prescription:
[0022] Sinomenine Hydrochloride 120.0g
[0024] Sucrose 50.8g
[0025] 2% hydroxypropyl methylcellulose aqueous solution 60g
[0026] Magnesium Stearate 5.8g Film Coated Prescription:
[0027] Cellulose acetate 80g
[0028] Polyethylene Glycol-4000 23g
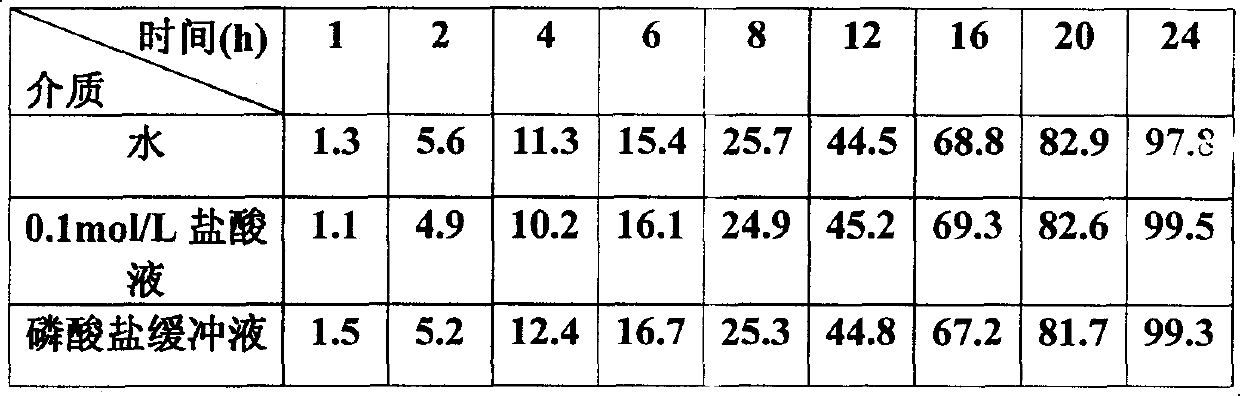
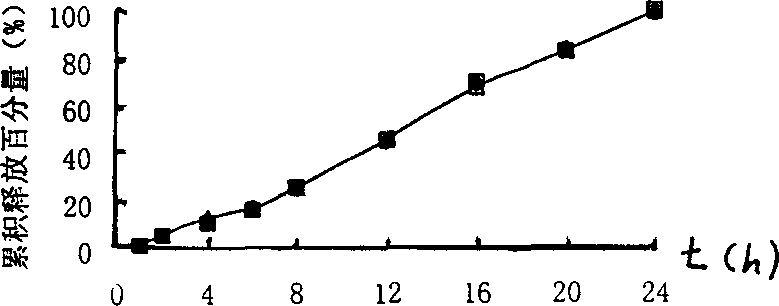
[0029] Preparation: Grind the prescription amount of medicine, sodium chloride and sucrose separately, and then sieve, mix evenly, add binder to make soft material, granulate with 20 mesh sieve, dry at 40±5°C, granulate with 16 mesh sieve, dry Granules plus lubricant magnesium stearate are pressed into tablet cores, cellulose acetate and plasticizer polyethylene glycol-4000 are dissolved in a mixed solvent of acetone and chloroform, the tablet cores are placed in a coating pan, and Coating, after the coating is completed, the coated tablet is dried in a drying oven to solidify the coating film. Then a dr...
Embodiment 2
[0031] Tablet prescription:
[0032] Sinomenine Hydrochloride 1200g
[0034] Sucrose 501g
[0035] 5% polyvinylpyrrolidone aqueous solution 400g
[0037] Coating film prescription:
[0038] Cellulose acetate 800g
[0039] Polyethylene Glycol-6000 200g
[0040] Preparation: Grind the prescribed amount of medicine, potassium chloride and sucrose separately, then sieve, mix evenly, add binder to make soft material, granulate with 20-mesh sieve, dry at 40±5°C, granulate with 16-mesh sieve, Dry granules plus lubricant magnesium stearate are pressed into tablet cores, cellulose acetate and plasticizer polyethylene glycol-6000 are dissolved in a mixed solvent of acetone and chloroform, and the tablet cores are placed in a coating pan. Coating is carried out, and after the coating is completed, the coated tablet is dried in a drying oven to solidify the coating...
Embodiment 3
[0042] Tablet prescription:
[0043] Sinomenine Hydrochloride 360g
[0044]Sodium chloride 330g
[0045] Sucrose 150g
[0046] 2% sodium carboxymethyl cellulose aqueous solution 150g
[0048] Coating film prescription:
[0049] Cellulose acetate 240g
[0050] Polyethylene Glycol-1500 90g
[0051] Preparation: Grind the prescribed amount of medicine, potassium chloride and sucrose separately, then sieve, mix evenly, add binder to make soft material, granulate with 20-mesh sieve, dry at 40±5°C, granulate with 16-mesh sieve, Dry granules plus lubricant magnesium stearate are pressed into tablet cores, cellulose acetate and plasticizer polyethylene glycol-1500 are dissolved in a mixed solvent of acetone and chloroform, and the tablet cores are placed in a coating pan. Coating is carried out, and after the coating is completed, the coated tablet is dried in a drying oven to solidify the coating f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com