Gene recombined medicine of adenovirus carrier and p53 gene for treating proliferative diseases
An adenovirus and recombinant technology, applied in the field of genetic engineering, can solve problems such as low transfection efficiency, low titer of retrovirus reproduction, and reduced immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
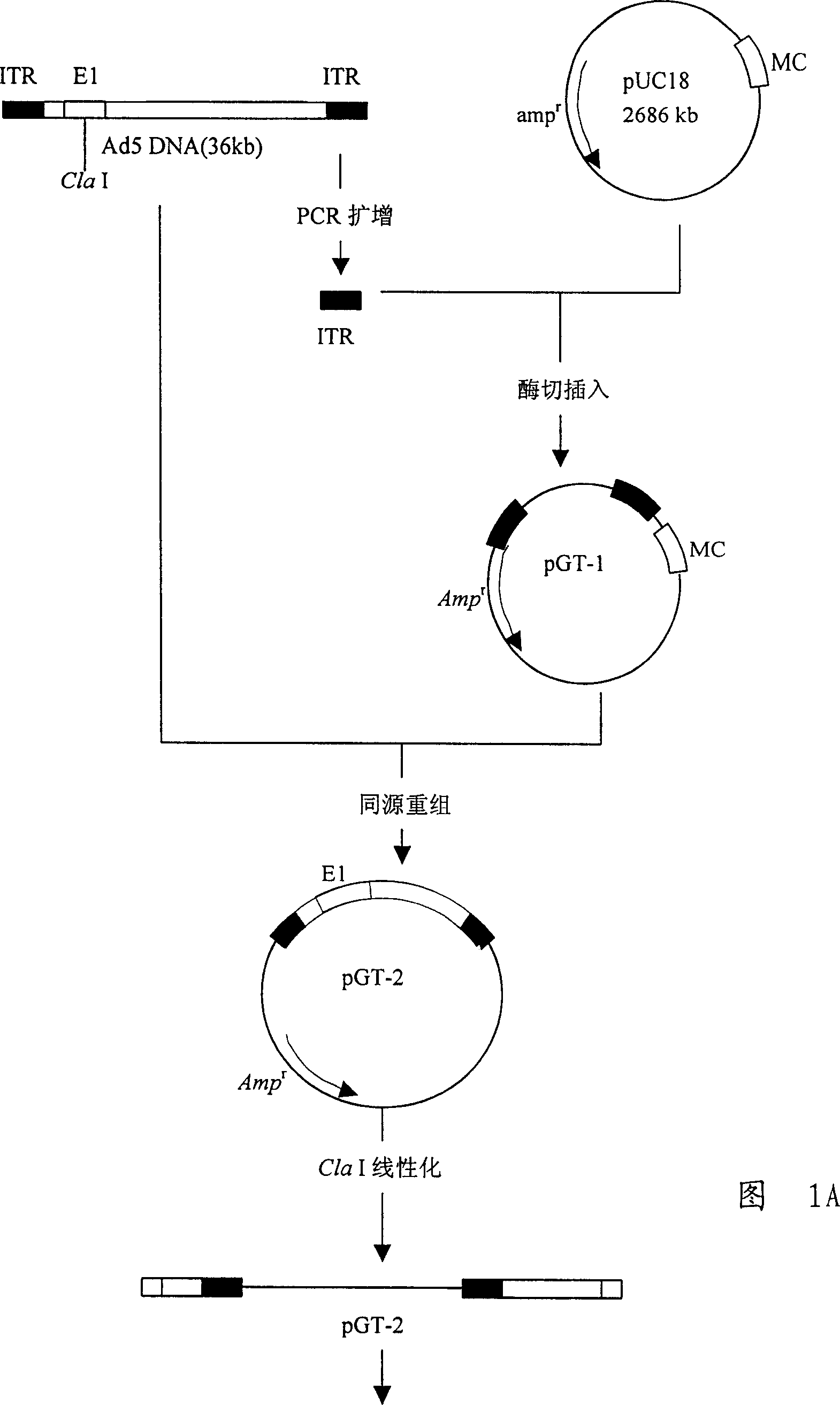
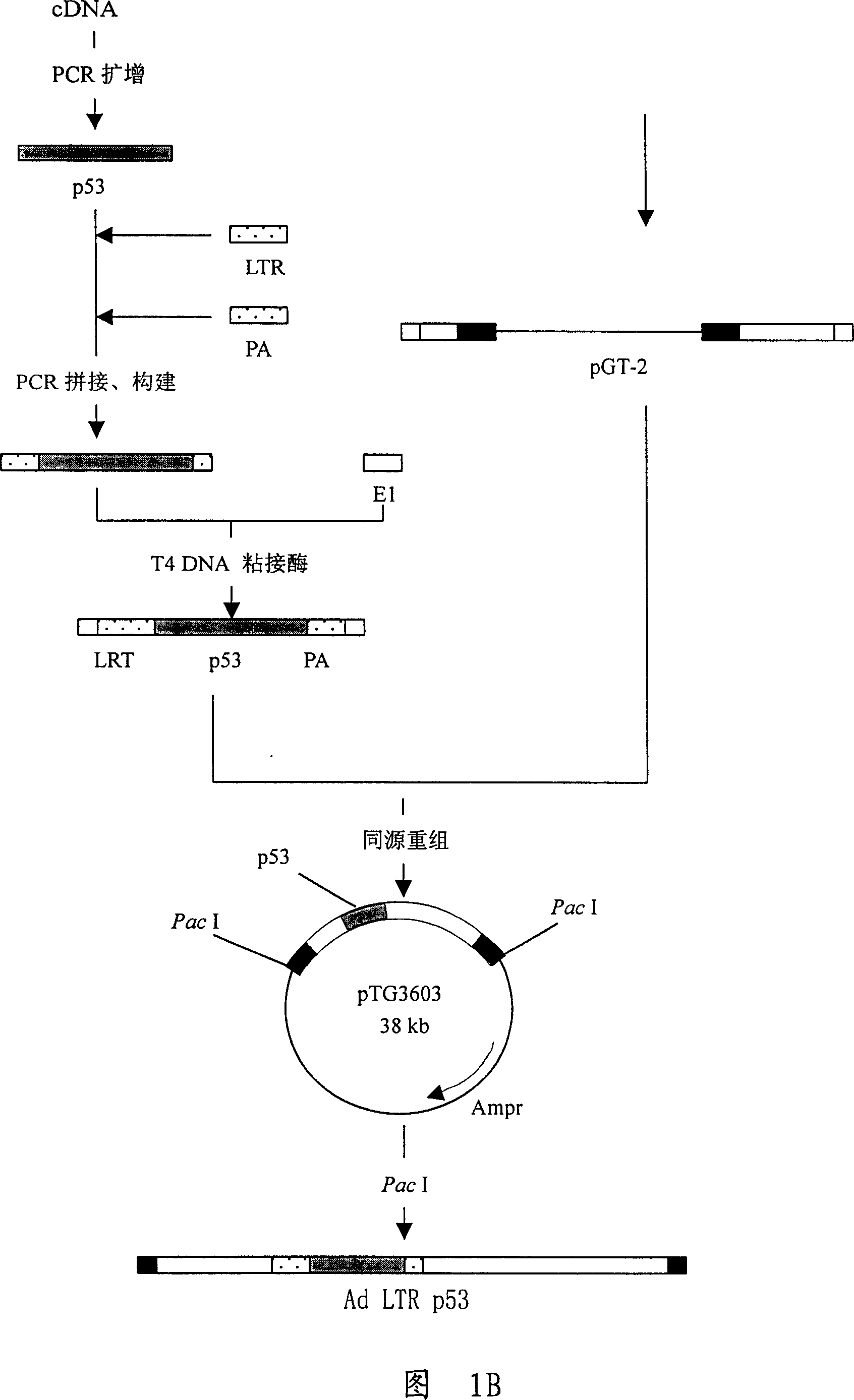
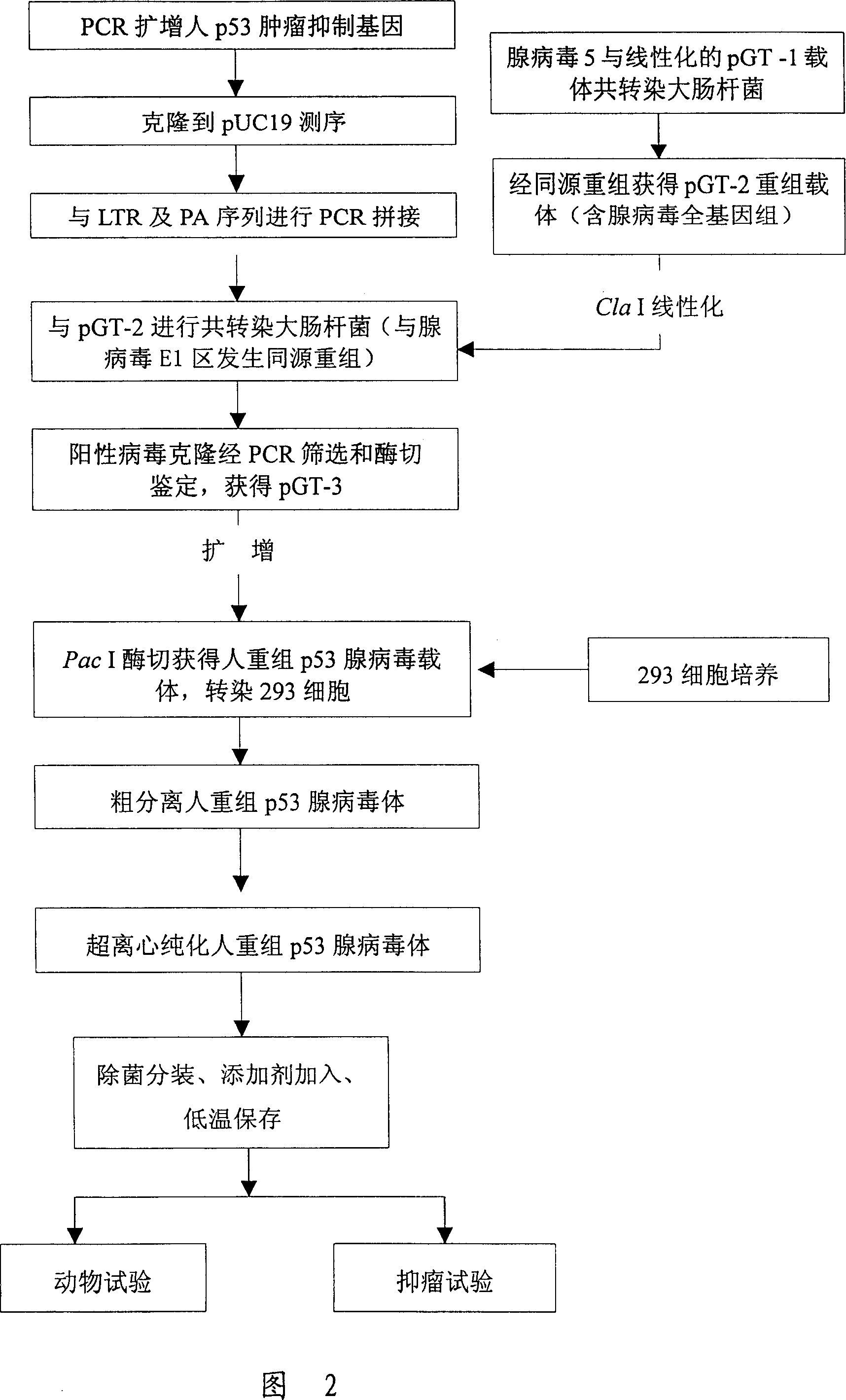
[0091] As shown in Figure 1 and Figure 2, construct gene recombinants and identify
[0092] 1. The published full cDNA sequence of p53 gene, design and synthesis of two primers:
[0093] 5'ATGGAGGAGCCGCAGTCAGATC and 5'ATATCTGCAGAATTCCAGCAC were used as primers, and linker sequences were introduced at both ends. Using HeLa cell cDNA as a template, the human p53 gene was amplified by PCR. The reaction conditions were as follows: the first cycle: denaturation at 94°C for 4 minutes, annealing at 58°C for 1 minute, and extension at 72°C for 2 minutes; subsequent cycles: 94°C Denaturation at °C for 1 minute, annealing at 58°C for 1 minute, extension at 72°C for 2 minutes, a total of 30 cycles. A large number of p53 genes were thus obtained, analyzed by agarose gel electrophoresis, and the full length of the p53 gene was recovered. After the fragment was purified, the p53 gene was cut with this enzyme, inserted into the pUC19 vector cut with the same enzyme for sequencing, and the c...
Embodiment 2
[0105] Killing effect of gene recombinants on primary cultured fibroblasts in vitro:
[0106] In vitro culture of scar fibroblasts (see Figure 6): the surgically excised scar skin was cut into 0.5-1cm under sterile conditions 3 Put the small pieces into the culture solution containing 1000U / ml penicillin and streptomycin immediately. First wash the tissue twice with PBS (containing penicillin and streptomycin) to remove fat and connective tissue, then wash it several times with D-Hank solution until the liquid has no oil droplets and is not turbid, then cut it to 1mm repeatedly 3 Add a few drops of serum to the tissue pieces, then place the skin pieces on the wall of the culture bottle at appropriate intervals, turn the culture bottle over so that the side with the tissue pieces faces up, add DMEM culture solution containing 10% FBS (note : Do not let the tissue come into contact with the culture medium!), place the side with the tissue block up, put it in a 37°C, 5% CO2 incu...
Embodiment 3
[0113] The therapeutic effect of the gene recombinant on keloid in the clinical research of gene therapy for scar.
[0114] As shown in Figure 10A and Figure 10B, a female patient with keloid had surgery on the left chest due to post-acne scar. Volume 2×1×1cm 3(See Figure 10A). After 4 weeks of gene therapy, the anterior chest scar shrunk significantly, its volume shrank significantly, and local tissues became dark (see Figure 10B). Except for self-limited fever, no other obvious toxic side effects were observed. The results of clinical trials show that gene recombinants are safe and effective in treating keloids.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com