Colon-released preparation of Melocicon and beta-cyclodextrin or its derivative composition
A technology for meloxicam and colon localization, which is applied to the colon localized release preparations of meloxicam and β-cyclodextrin or its derivatives, and the field of meloxicam colon localized release formulations, which can solve the problem of weakening individual differences. , drug efficacy uncertainty, difficulties and other issues, to achieve the effect of increasing solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
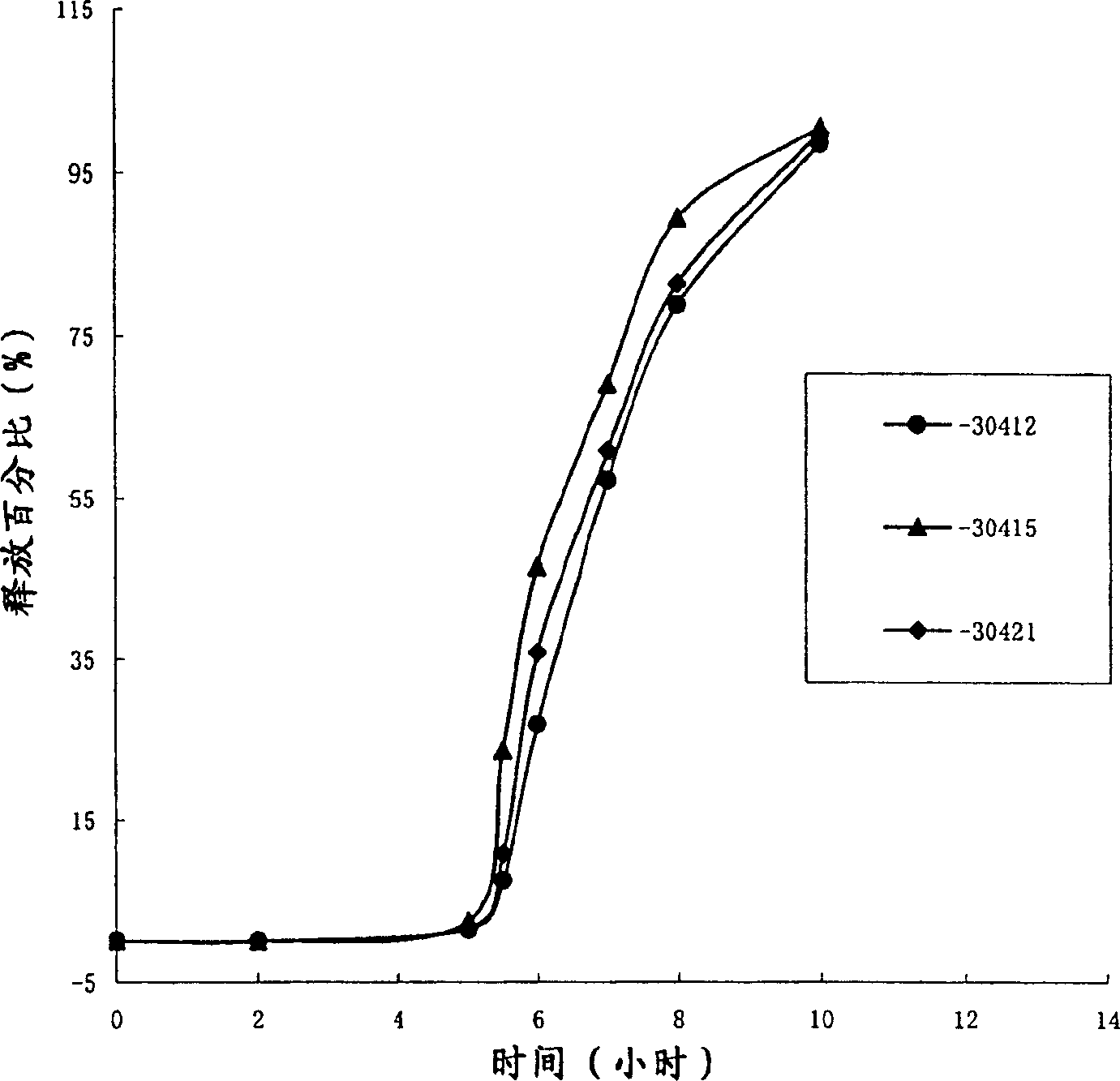
Image
Examples
Embodiment 1
[0040] Example 1 Meloxicam Colon-targeted Release Capsules of 7.5mg Specification
[0041] Take 75g of β-cyclodextrin, add 780mL of water, heat to dissolve at 80°C, add 25g of meloxicam passed through a 100-mesh sieve, add about 2mL of ammonia water, make the pH value 7.5, stir for 4 hours, and dissolve. Freeze-dry to obtain a solidified product of the meloxicam / β-cyclodextrin complex. Get 100g pectin, 170g lactose as diluent, 30g microcrystalline cellulose as disintegrant, use 5% HPMC (6cps) as binding agent to granulate, cross 16 mesh sieves, dry at 60°C for 2 hours, pass 20 mesh Sieve the granules, add 1% magnesium stearate and mix. Select No. 3 B-type enteric-coated capsules (colon-positioned release capsules) for filling. A new formulation of meloxicam colon-targeted release capsules with a specification of 7.5 mg was obtained.
Embodiment 2
[0042] Example 2 7.5 mg of meloxicam colon-targeted release matrix tablet
[0043] Take 75g of β-cyclodextrin, add 780mL of water, heat to dissolve at 80°C, add 25g of meloxicam passed through a 100-mesh sieve, add about 2mL of ammonia water, make the pH value 7.5, stir for 4 hours, and dissolve. Freeze-dry to obtain a solidified product of the meloxicam / β-cyclodextrin complex. Get 100g pectin, 170g lactose as diluent, 30g microcrystalline cellulose as disintegrant, use 5% HPMC (6cps) as binding agent to granulate, cross 16 mesh sieves, dry at 60°C for 2 hours, pass 20 mesh Sieve the granules, add 1% magnesium stearate and mix. Select a concave die with a diameter of 7mm, adjust the pressure to 5-7kg, and press the tablet. A new formulation of meloxicam colon-targeted release matrix tablet with a specification of 7.5 mg was obtained.
Embodiment 3
[0044] Example 3 Preparation of 10 mg meloxicam colon-targeted pellets
[0045] Take 75g of β-cyclodextrin, add 780mL of water, heat to dissolve at 80°C, add 25g of meloxicam passed through a 100-mesh sieve, add 2mL of ammonia water, make the pH value 7.5, stir for 4 hours, and dissolve. Add 5g of hydroxypropylmethylcellulose (12cps) as a binder, stir and dissolve at 40°C, add 5g of talcum powder as an anti-sticking agent, stir to form a uniform suspension, and set aside. Take 500 g of blank sugar pills with a diameter of 810 μm, place them in a fluidized bed (Glatt GPCG1) bottom spray container, select a nozzle with a diameter of 1.0 mm, turn on the fluidization switch, adjust the fluidization state, and preheat the material to about 30 ° C; heat Apply the drug coating solution to 30°C. Set the parameters of the fluidized bed as air inlet temperature 45°C, air inlet valve 50%, atomization pressure 1.5bar, spray rate 5ml / min, the inlet air temperature after the state is stabl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com