Method for fermenting recombinant protein by micro-aerobic induction of escherichia coli
A technology of Escherichia coli and protein, applied in the field of recombinant protein biological products, can solve the problems of low price, excessive protein expression, low induction strength, etc., achieve the effect of reducing requirements, simplifying fermentation equipment, and facilitating later amplification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) Construction of strains
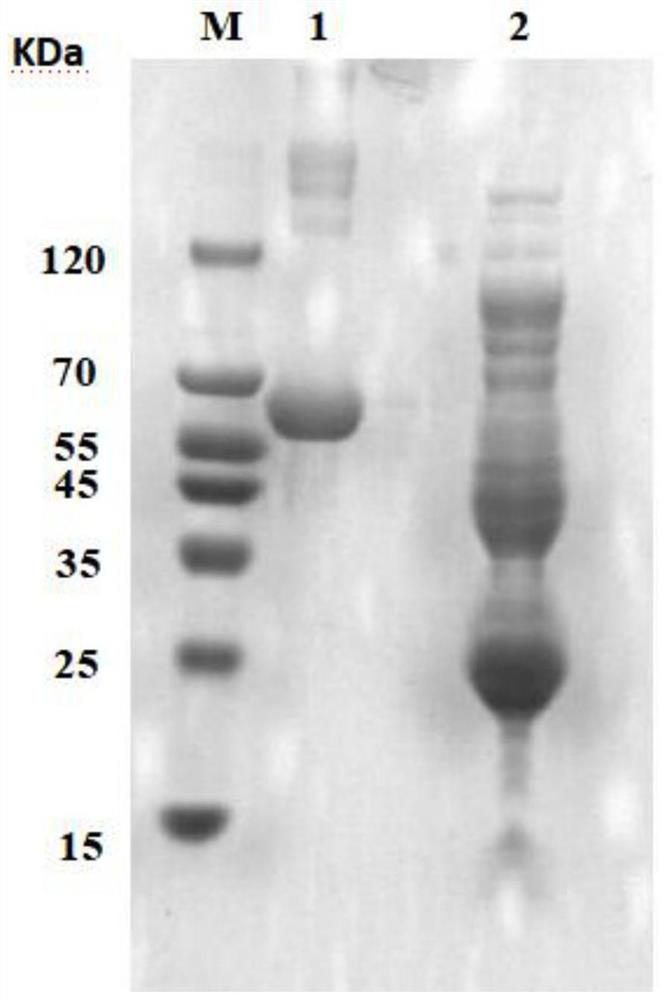
[0037] Using Escherichia coli BL21(DE3) strain, 6 histidine tags were introduced at the end of the human-like collagen protein gene, and then connected to the vector pET-24a(+) to construct the expression plasmid col-pET-24a(+), which will be verified correctly The recombinant plasmid was transformed into E. coli competent cells BL21 (DE3), and the genetically engineered bacteria E expressing recombinant human-like collagen were all positive for LB plate identification containing kanamycin, colony PCR identification and sequencing identification. The .coil BL21(DE3) / pET-24a(+)-col strain was stored in a -80°C refrigerator until use.
[0038]Specifically, the recombinant human-like collagen base sequence of the above-mentioned recombinant human-like collagen genetically engineered bacteria is artificially composed of an eight-repeat type I human collagen sequence fragment and a type III human collagen sequence fragment. Splicing synthesis (...
Embodiment 2
[0059] (1) Construction of strains
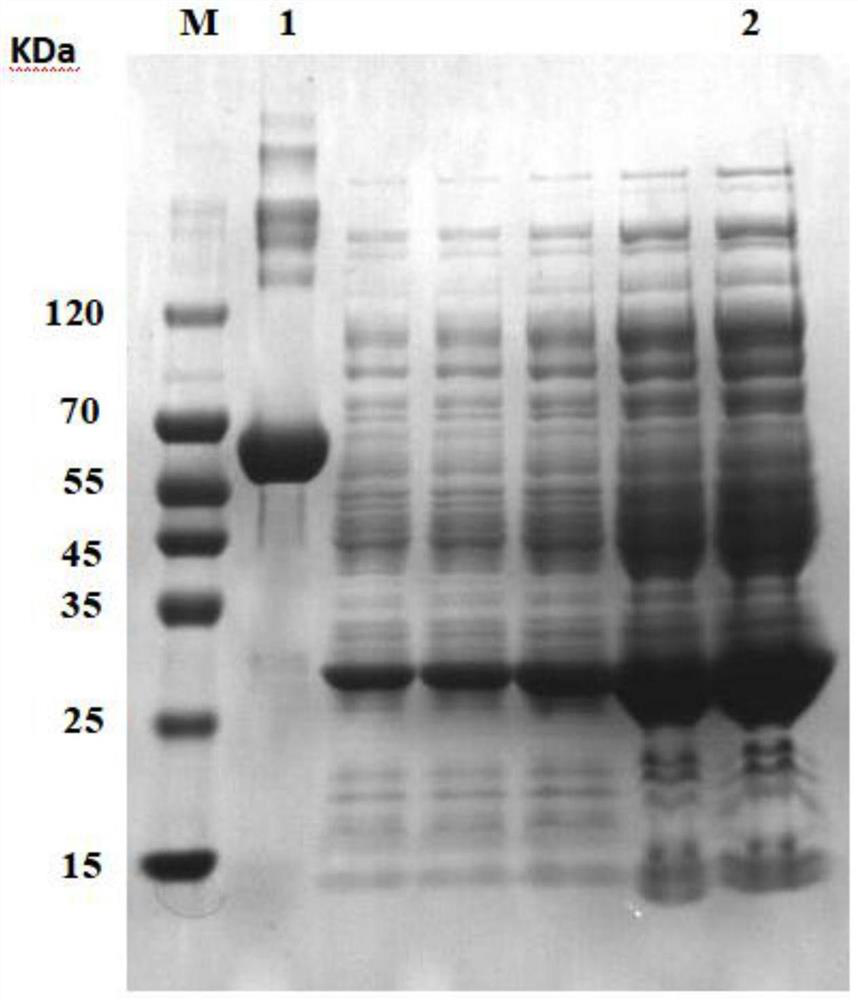
[0060] Using Escherichia coli BL21(DE3) strain, 6 histidine tags were introduced at the end of the collagen gene, and then connected to the vector pET-24a(+) to construct the expression plasmid col-pET-24a(+), and the correct recombinant plasmid will be verified. It was transformed into E. coli competent cells BL21 (DE3), and the genetically engineered bacteria E.coil BL21 expressing recombinant human collagen was positive for the identification of LB plate containing kanamycin, colony PCR identification and sequencing identification. (DE3) / pET-24a(+)-col strain was stored in a -80°C refrigerator until use.
[0061] Specifically, the recombinant human-like collagen base sequence of the above-mentioned recombinant human-like collagen genetically engineered bacteria is artificially composed of an eight-repeat type I human collagen sequence fragment and a type III human collagen sequence fragment. Splicing synthesis (refer to the prior art fo...
Embodiment 3
[0082] (1) Construction of strains
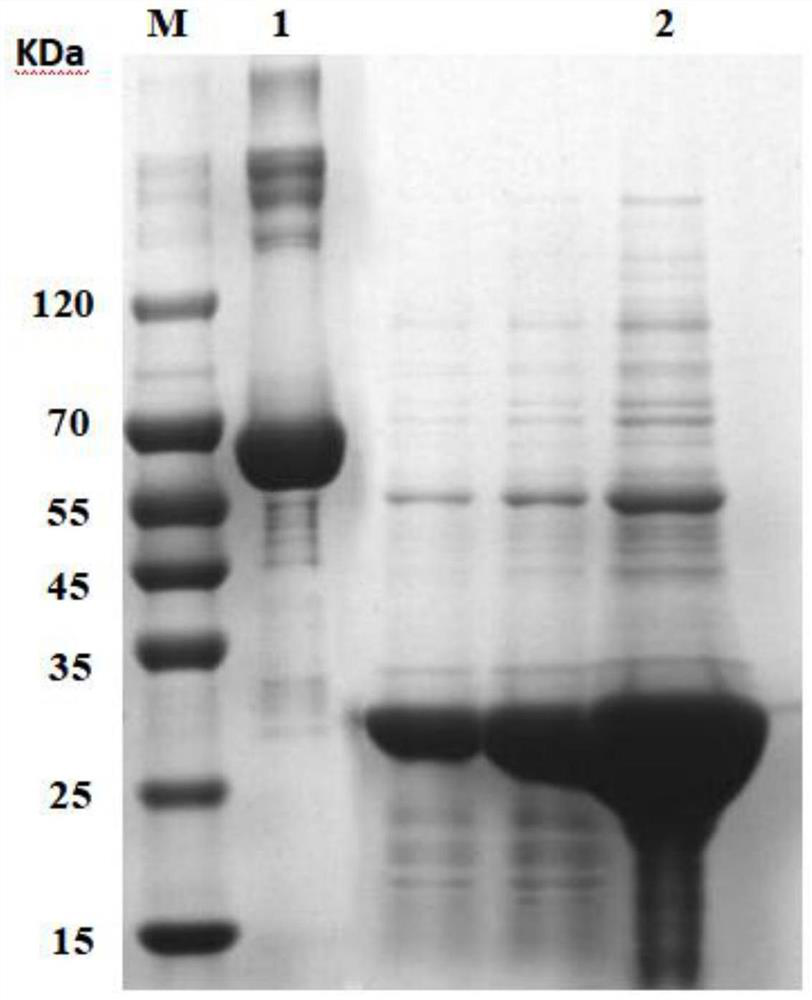
[0083] Using Escherichia coli BL21(DE3) strain, 6 histidine tags were introduced at the end of the collagen gene, and then connected to the vector pET-24a(+) to construct the expression plasmid col-pET-24a(+), and the correct recombinant plasmid will be verified. It was transformed into E. coli competent cells BL21 (DE3), and the genetically engineered bacteria E.coil BL21 expressing recombinant human collagen was positive for the identification of LB plate containing kanamycin, colony PCR identification and sequencing identification. (DE3) / pET-24a(+)-col strain was stored in a -80°C refrigerator until use.
[0084]Specifically, the recombinant human-like collagen base sequence of the above-mentioned recombinant human-like collagen genetically engineered bacteria is artificially composed of an eight-repeat type I human collagen sequence fragment and a type III human collagen sequence fragment. Splicing synthesis (refer to the prior art for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com