Process for preparing tricyclodecane dimethylamine from dicyclopentadiene and application of tricyclodecane dimethylamine
A technology of dicyclopentadiene and decane dimethylamine, applied in the field of IPCC07C, can solve the problems of tricyclodecane diformaldehyde thermal instability, high overall cost, catalyst loss, etc., achieve good industrial application prospects and improve reaction Depth, the effect of avoiding yield loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
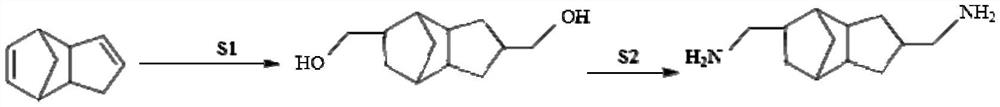
[0051] The present embodiment provides a process for preparing tricyclodecane dimethanol with dicyclopentadiene, and the process steps are specifically:
[0052] In a 250ml autoclave, add 60g of solvent, 50g of dicyclopentadiene, 0.36g of metal catalyst, 0.1g of tris(2,4-di-tert-butyl) phenyl phosphite, seal the reactor, and use nitrogen and synthesis gas in turn Each autoclave was purged three times, filled with synthesis gas, and the temperature was increased. The conditions in the control reactor were kept at 120°C and 6MPa. The reaction was terminated after 6 hours of reaction, the temperature was lowered and the pressure was lowered, the synthesis gas was vented, and the obtained product was distilled to obtain 62 g of tricyclodecane dimethanol with a yield of 84%.
[0053] The solvent is tetrahydrofuran.
[0054] The catalyst is cobalt acetylacetonate and ruthenium trichloride with a weight ratio of 35:1.
[0055] The ligand is tris(2,4-di-tert-butyl) phenyl phosphite...
Embodiment 2
[0058] The present embodiment provides a process for preparing tricyclodecane dimethanol with dicyclopentadiene, and the process steps are specifically:
[0059] In a 1000ml autoclave, add 300g solvent, 300g dicyclopentadiene, 1.64g metal catalyst, 1.1g ligand, seal the autoclave, use nitrogen and syngas to purge the autoclave three times in turn, fill with syngas to start Heat up and boost. The conditions in the control reactor were kept at 110°C and 8MPa. After the reaction for 8 hours, the reaction was terminated, the temperature was lowered and the pressure was lowered, the synthesis gas was vented, and the obtained product was distilled to obtain 347 g of tricyclodecane dimethanol with a yield of 78%.
[0060] The solvent is toluene.
[0061] The catalyst is cobalt chloride and carbonyl ruthenium chloride in a weight ratio of 15:1.4.
[0062] The ligand is triphenylphosphine.
[0063] The synthesis gas is a combination of carbon monoxide and hydrogen; the volume ratio...
Embodiment 3
[0065] The present embodiment provides a process for preparing tricyclodecane dimethanol with dicyclopentadiene, and the process steps are specifically:
[0066] In a 2000ml autoclave, add 500g solvent, 550g dicyclopentadiene, 3.92g metal catalyst, 0.9g ligand, seal the autoclave, use nitrogen and syngas to purge the autoclave three times in turn, fill with syngas to start Heat up and boost. The conditions in the control reactor were kept at 120°C and 6MPa. The reaction was terminated after 6 hours of reaction, the temperature was lowered and the pressure was lowered, the synthesis gas was vented, and the obtained product was distilled to obtain 661 g of tricyclodecane dimethanol with a yield of 81%.
[0067] The solvent is tetrahydrofuran.
[0068] The catalyst is cobalt acetylacetonate and ruthenium trichloride in a weight ratio of 38.5:0.7.
[0069] The ligand is tris(2,4-di-tert-butyl) phenyl phosphite.
[0070] The synthesis gas is a combination of carbon monoxide and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com