Blood-brain barrier penetrating drug delivery system as well as preparation method and application thereof
A blood-brain barrier permeation and delivery system technology, applied in the field of blood-brain barrier drug delivery system and its preparation, can solve the problems of cytotoxicity and difficult clinical transformation, and achieve high-efficiency permeation of the blood-brain barrier and excellent anti-glioma effect. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] In this example, a neutrophil exosome is extracted, and the method is as follows:
[0060] (1) Take 25g Kunming mice, kill them by neck dislocation after anesthesia, put them in 75% ethanol for sterilization for 5min, and move them into an ultra-clean workbench;
[0061] (2) Carefully separate the mouse leg skin with sterilized surgical equipment, cut out the entire thigh, put it in pre-cooled PBS, peel off the mouse leg muscle, take out the femur and tibia, and put it into the femur and tibia containing RPMI1640 the petri dish;
[0062] (3) Rinse out the bone marrow with a fresh amount of RPMI1640, filter the cells with a 70 μm filter, centrifuge at 450 × g for 10 min, and resuspend the pellet in PBS solution;
[0063] (4) Use erythrocyte lysate to lyse erythrocytes, centrifuge at 450 × g for 5 min, and resuspend the pellet in PBS solution to obtain a single-cell suspension;
[0064] (5) Prepare percoll stock solution with 10×PBS and percoll cell separation solution ...
Embodiment 2
[0070] The present embodiment prepares a blood-brain barrier drug delivery system, and the method is as follows:
[0071] (1) Use BCA kit to measure neutrophil exosome protein concentration, take 0.4 mg / mL neutrophil exosomes extracted according to the method of Example 1 (exosomes diluted with 1×PBS, 0.22 μm Use after filtration with a sterile filter), add an equivalent concentration of 0.4 mg / mL DOX solution (DOX is prepared with 1×PBS containing 5% DMSO);
[0072] (2) Put the mixture of neutrophil exosomes and DOX in a centrifuge tube, and perform sonication in an ultrasonic cell disruptor; ultrasonic conditions: 20% amplitude, 6 cycles, 4s on, 2s off, each Cool the samples on ice for 2 min between cycles;
[0073] (3) After the treatment, the samples were incubated at 37°C for 1 h to ensure the recovery of the plasma membrane of NEs-Exos;
[0074] (4) After incubation at 37°C, the samples were centrifuged at 100,000 × g for 70 min, washed with PBS, and centrifuged again ...
Embodiment 3
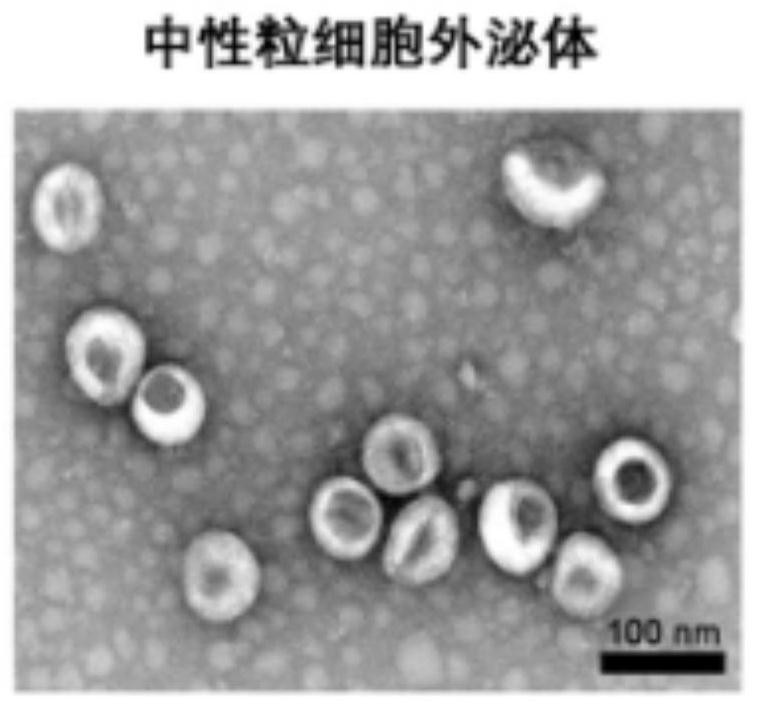
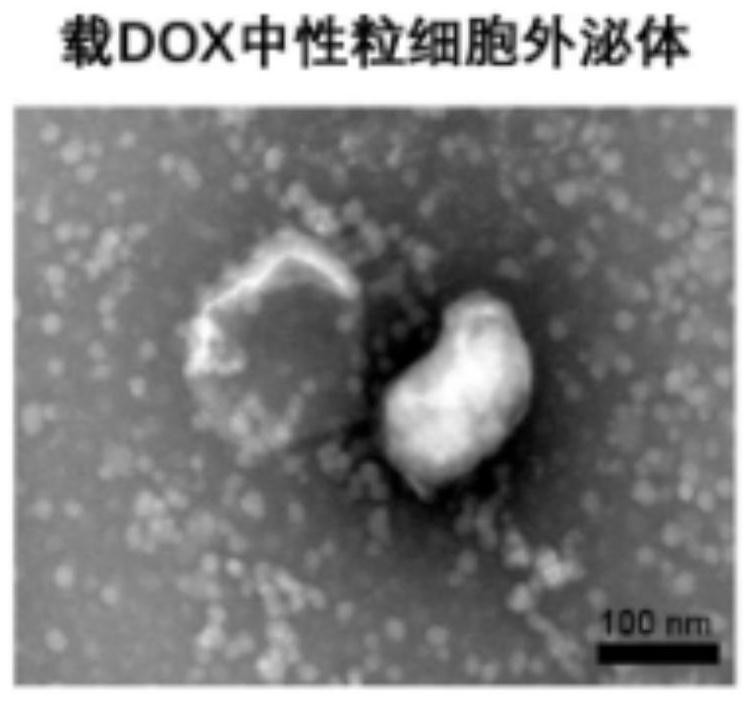
[0076] Observation of transmission electron microscopy on the neutrophil exosomes and blood-brain barrier drug delivery systems prepared in Example 1 and Example 2: combining neutrophil exosomes or blood-brain barrier drug delivery systems with etc. The amount of 4% paraformaldehyde was mixed and fixed on a sample-loaded copper grid (200 mesh, coated with a formaldehyde metal film). After 20min washing with PBS, the copper mesh was placed on a drop of 1% glutaraldehyde for 5min. The copper mesh was then washed in ultrapure water. 2min each time, a total of 8 times. Images were captured using a JEM 1200EX TEM, respectively as figure 1 and figure 2 As shown in the figure, it can be seen from the figure that the neutrophil exosomes are cup-shaped or saucer-shaped ( figure 1 ), while the particle size of neutrophil exosomes loaded with DOX increased, but the morphology was also consistent with the exosome morphology ( figure 2 ).
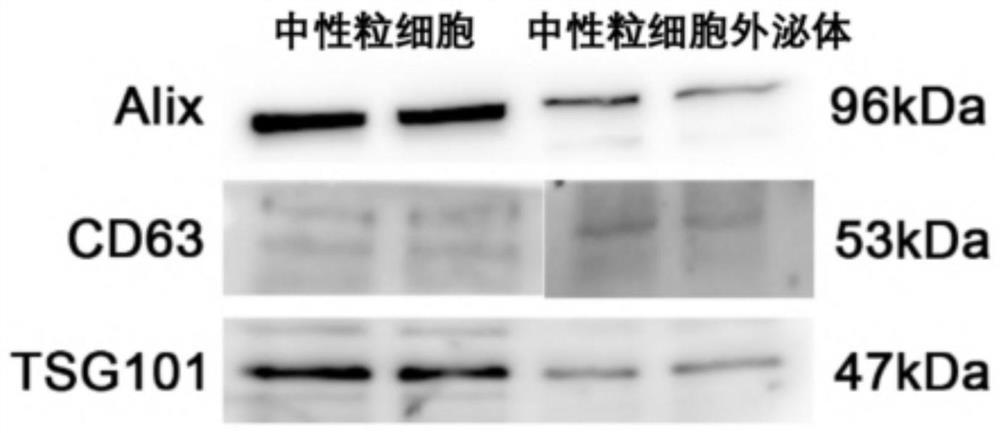
[0077] Western blot analysis was performe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com