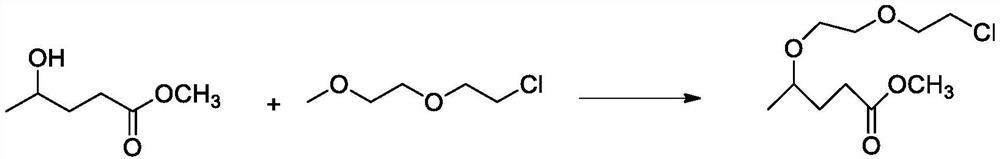
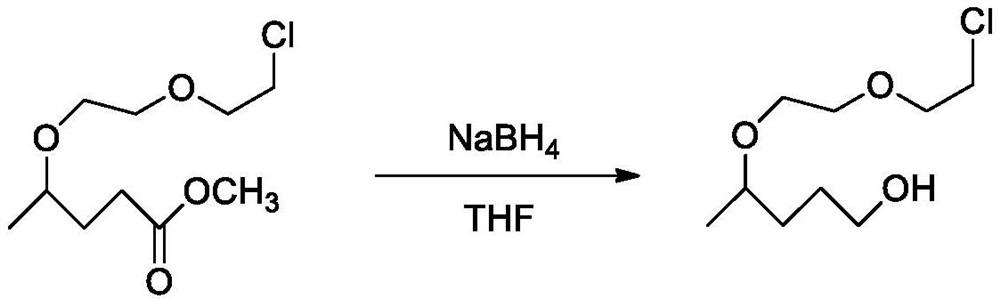
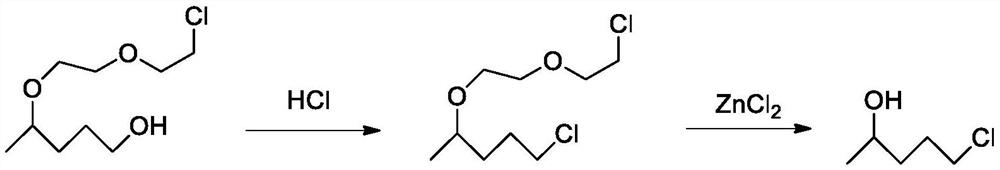
Synthesis process of 5-chloro-2-pentanone
A synthesis process and pentanone technology are applied in the field of synthesis technology of 5-chloro-2-pentanone, can solve the problems of high price of raw materials, harsh reaction conditions and high price of raw materials, achieve large interlayer distance and easy availability of raw materials , the effect of cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The composite catalyst comprises the following steps to prepare:
[0030] D1, will 1.57gZn (NO 3 ) 2 ·6H 2 O and 0.95gAl (NO 3 ) 3 ·9H 2 The mixture of O was dissolved in 45 g of deionized water, then 10.3 g of sodium salt solution of tetrakis (4-sulfonic acid phenyl) porphyrin cobalt was added, and the pH value was maintained at 8.5 by continuously dripping 18 g of 0.1 mol / L NaOH solution, Stir at 80°C for 24h, filter the product, wash with distilled water at 80°C, and finally dry at room temperature to obtain the catalyst precursor;
[0031] D2. After stirring and mixing 0.55 mmol of N-hydroxyphthalimide and 28 mg of the catalyst precursor uniformly, a composite catalyst is obtained.
Embodiment 2
[0033] The composite catalyst comprises the following steps to prepare:
[0034] D1. 1.67g Zn(NO 3 ) 2 ·6H 2 O and 1.05g Al (NO 3 ) 3 ·9H 2 The mixture of O was dissolved in 50 g of deionized water, and then 11.2 g of sodium salt solution of tetrakis(4-sulfonic acid phenyl) porphyrin cobalt was added, and the pH value was maintained at 8.5 by continuous dropwise addition of 21 g of 0.1 mol / L NaOH solution. Stir at 80°C for 24h, filter the product, wash with distilled water at 80°C, and finally dry at room temperature to obtain the catalyst precursor;
[0035] D2. After stirring and mixing 0.61 mmol of N-hydroxyphthalimide and 30 mg of the catalyst precursor uniformly, a composite catalyst is obtained.
Embodiment 3
[0037] The composite catalyst comprises the following steps to prepare:
[0038] D1. 1.72g Zn(NO 3 ) 2 ·6H 2 O and 1.15g Al (NO3 ) 3 ·9H 2 The mixture of O was dissolved in 55g of deionized water, then 12.5g of sodium salt solution of tetrakis (4-sulfonic acid phenyl) porphyrin cobalt was added, and the pH value was maintained at 8.5 by continuous dropwise addition of 25g of 0.1mol / L NaOH solution, Stir at 80°C for 24h, filter the product, wash with distilled water at 80°C, and finally dry at room temperature to obtain the catalyst precursor;
[0039] D2. After stirring and mixing 0.64 mmol of N-hydroxyphthalimide and 32 mg of catalyst precursor uniformly, a composite catalyst is obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com