Functionalized polystyrene polymer as well as preparation method and application thereof
A polystyrene and polystyrene technology, applied in the field of functionalized polystyrene polymers and their preparation, can solve the problems of expensive, harsh reaction conditions, unsatisfactory site selectivity, etc., and achieve low cost, high reaction mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050]
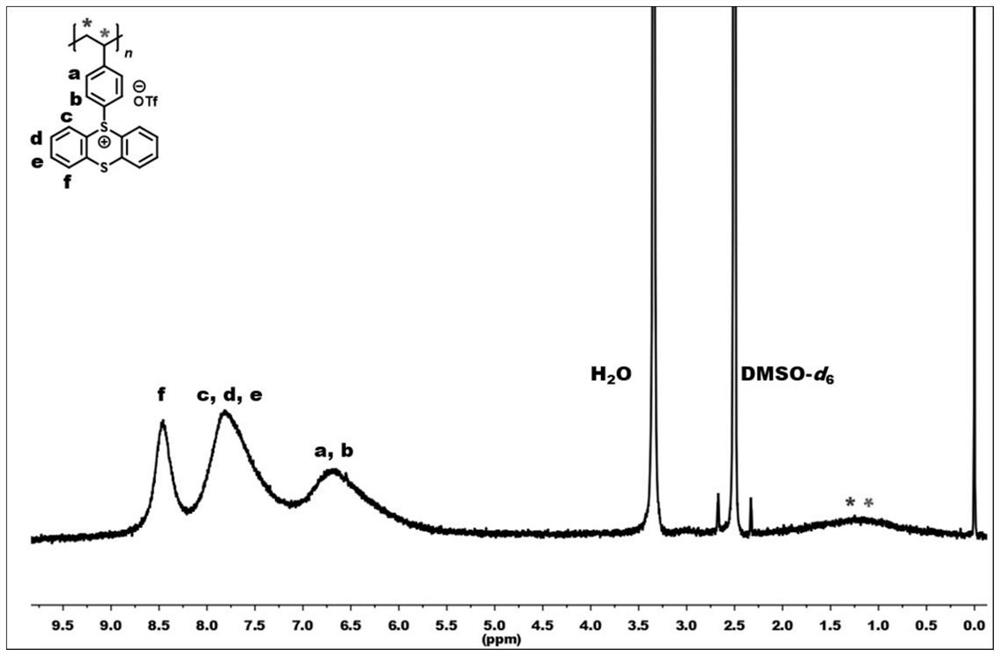
[0051] Under air atmosphere, polystyrene (1340mg, 10mmol) (n=954) and thianthrene sulfoxide (2320mg, 10mmol) were dissolved in 40mL of dichloromethane, and trifluoromethanesulfonic anhydride (8460mg , 30mmol), mix well, slowly rise to room temperature and react for 24h, after the reaction is completed, the reaction solution is diluted (1mL dichloromethane), then sinks into ethyl acetate, filters, washes with water, filters, and vacuum-dries to obtain formula II Polystyrene thianthracene salt represented by -1 (pale yellow solid product, 4446 mg, yield 95%). figure 1 It is a schematic diagram of the hydrogen nuclear magnetic resonance spectrum of the polystyrene thianthracene salt prepared in Example 1 of the present invention. 1 H NMR (400MHz, DMSO-d 6 )δ8.46(s,2H),7.80(s,6H),6.69(s,4H),2.98(s,1H),1.24(s,2H).
[0052] Under nitrogen atmosphere, dissolve the polystyrene thianthracene salt (468mg, 1mmol) represented by formula II-1 in 4mL N,N-dimethylformamide, add...
Embodiment 2
[0054]
[0055] Under nitrogen atmosphere, polystyrene thianthracene salt (468 mg, 1 mmol) represented by formula II-1 was dissolved in 4 mL of N,N-dimethylformamide, p-chlorophenylboronic acid (188 mg, 1.2 mmol), Sodium bicarbonate (252mg, 3mmol) and bis(tri-tert-butylphosphine)palladium (25mg, 0.05mmol) were mixed uniformly, reacted at room temperature for 12h, after the reaction was completed, concentrated in turn, precipitated in methanol, filtered, washed with water, After filtration and vacuum drying, the functionalized polystyrene polymer represented by formula I-2 was obtained (gray solid product, 189 mg, yield 88%). 1 H NMR (400MHz, Chloroform-d) δ7.26(d, J=1.8Hz, 6H), 6.54(s, 2H), 1.80(s, 1H), 1.47(s, 2H).
Embodiment 3
[0057]
[0058] Under nitrogen atmosphere, polystyrene thianthracene salt (468mg, 1mmol) represented by formula II-1 was dissolved in 4mL N,N-dimethylformamide, and 4-methoxyphenylboronic acid (182mg, 1.2 mmol), sodium bicarbonate (252mg, 3mmol), bis(tri-tert-butylphosphine) palladium (25mg, 0.05mmol) were mixed uniformly, reacted at room temperature for 12h, after the reaction was completed, concentrated successively, precipitated in methanol, filtered , washed with water, filtered, and vacuum-dried to obtain a functionalized polystyrene polymer represented by formula I-3 (light yellow solid product, 189 mg, yield 90%). 1 H NMR (400MHz, Chloroform-d) δ7.26(s,4H),6.80(s,2H),6.58(s,2H),3.78(s,3H),1.88(s,1H),1.68-1.16( m,2H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com