Antibacterial peptide YHX-4 and application thereof
A YHX-4, antibacterial peptide technology, applied in applications, antibacterial drugs, peptides, etc., to achieve the effects of small molecular weight, short synthetic sequence, and low difficulty in chemical synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The design of embodiment 1 antimicrobial peptide
[0031] Based on the de novo design of antimicrobial peptides and the understanding of the structure-activity relationship, the sequence parameters of 843 antimicrobial peptide sequences screened from the ADP3 database with antibacterial effects on Gram-positive and Gram-negative bacteria were analyzed ( Including sequence length, charged number, hydrophobic amino acid ratio and amino acid composition), and then according to the principle of superior parameter selection and combined with rational design ideas, the sequence parameters of the new antimicrobial peptide were determined, as follows:
[0032] Table 1 Sequence parameters of de novo designed antimicrobial peptides
[0033]
[0034] In order to reduce the synthesis cost and reduce the cytotoxicity, the sequence length was selected as 13, which is the second most frequently used sequence. The positively charged amino acid Lys and the polar uncharged amino acid...
Embodiment 2
[0044] Embodiment 2 antibacterial activity
[0045] Antimicrobial peptides were synthesized by Qiangyao Biotechnology (Shanghai) Co., Ltd.
[0046] Staphylococcus aureus, Escherichia coli and Salmonella were respectively inoculated on LB solid medium, Listeria monocytogenes and Streptococcus mutans were inoculated on BHI solid medium, and cultured in a constant temperature incubator at 37°C After 18 hours, a single colony of each strain was picked and placed in their corresponding liquid medium, and cultured with shaking at a constant temperature of 37°C for 12 hours. Measure the OD of the bacterial solution 600 value, and dilute it to 1×10 6 CFU / mL.
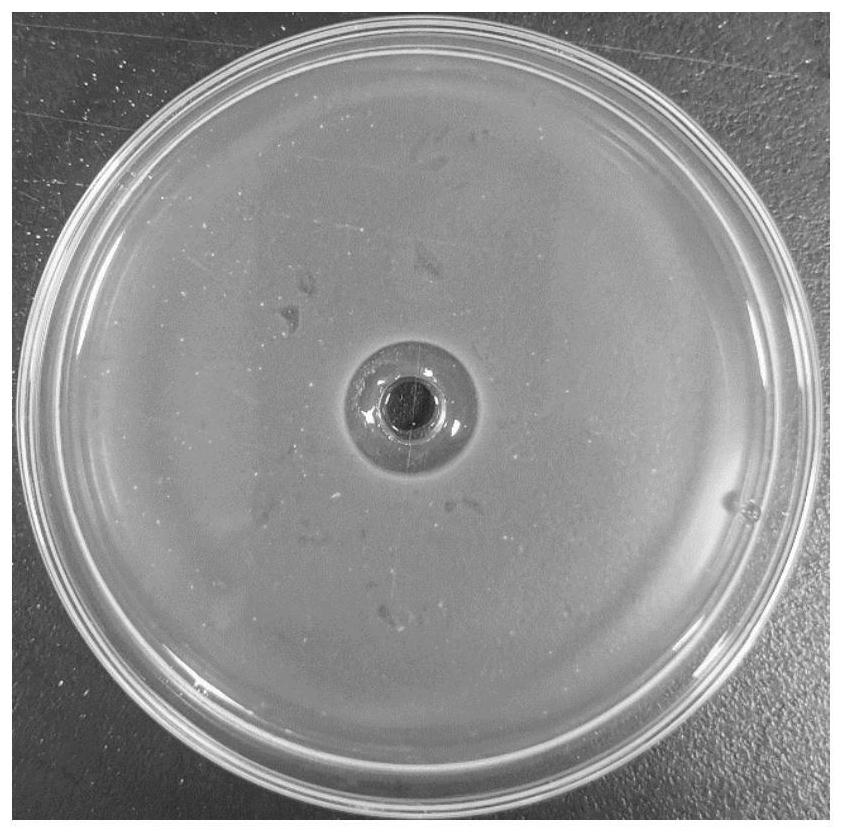
[0047] ①Inhibition zone test
[0048] Prepare LB and BHI semi-solid medium (mass fraction of agar 0.6%), add 7 μL of bacterial solution to 20mL of each plate, shake and mix well, pour it into the petri dish with Oxford cup placed, and pull out the Oxford cup after the medium is cooled and solidified to complete the infusion....
Embodiment 3
[0053] Example 3 Hemolytic activity
[0054] Take 1mL of healthy rabbit blood and add it to a heparin anticoagulant tube, centrifuge at 1000xg for 10min, get the precipitate, wash with PBS buffer 3 times, and resuspend the red blood cells in 10mL of PBS. Adjust the concentration of antimicrobial peptide YHX-4 to 4-512 μg / mL with PBS buffer, add 50 μL per well into a 96-well plate, and add an equal volume (50 μL) of red blood cell suspension and mix well. Take PBS buffer as a negative control, and 0.1% Tritonx-100 as a negative control, take it out after incubating at 37°C for 1 hour, centrifuge at 1000xg for 10 minutes, take out the supernatant and measure the OD value at 570nm with a microplate reader, and the test results are shown in the table 4.
[0055] The calculation formula of hemolysis rate is: hemolysis rate=(A T -A O ) / (A C -A O )×100%.
[0056] In the formula: A T is the absorbance value of the experimental group, A C is the absorbance value of the positive...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com