Bispecific chimeric antigen receptor targeting HIV-1 (human immunodeficiency virus-1) envelope protein as well as preparation method and application of bispecific chimeric antigen receptor
A chimeric antigen receptor and HIV-1 technology, applied in the field of immunotherapy, can solve the problem of affecting the expression level of CAR molecular membrane, unfavorable control of HIV-1 infection, and weakening the ability of CAR-T cell activation to kill HIV-1 infected target cells capacity and other issues to achieve the effect of increasing clinical effectiveness, improving broad-spectrum and specificity, and improving survival
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 Construction of lentiviral expression plasmid The M31 CAR and M13CAR genes (shown in SEQ ID No:8 and SEQ ID No:9, respectively) were synthesized by Shanghai Jierui Bioengineering Co., Ltd., and cloned into blank lentiviral expression plasmids (pKL) to obtain pKL-M31-CAR, respectively and pKL-M13-CAR recombinant lentiviral expression plasmid, bispecific chimeric antigen receptor structure such as figure 1 and 2 shown.
Embodiment 2
[0047] Packaging, concentration and titer determination of embodiment 2 lentivirus
[0048] 1.1 Packaging of lentivirus
[0049] Treatment of HEK293T cells: 24 hours before transfection, HEK293T cells in logarithmic growth phase were collected and seeded in 10 cm cell culture dishes (6-8×10 6 cells), the cells were grown in 10 mL of complete DMEM medium at 37 °C, 5% CO 2 Culture under the conditions for 18-24 hours, and the cell density can reach above 70-90%, and then transfection can be carried out.
[0050] HEK293T cell transfection: Add 1mL basal DMEM medium in a 15mL centrifuge tube, according to the mass ratio: lentiviral expression plasmid (pKL-M31-CAR or pKL-M13-CAR): packaging plasmid psPAX2: envelope plasmid PMD2.G= Prepare the transfection mixture at 1:3:1, and the total amount of plasmids is 15 μg / dish. Add 30 μL of TurboFect transfection reagent at a ratio of plasmid amount (μg):transfection reagent (μL)=1:2, incubate at room temperature for 15-20 minutes, ad...
Embodiment 3
[0054] Infection and expansion of embodiment 3 T cells
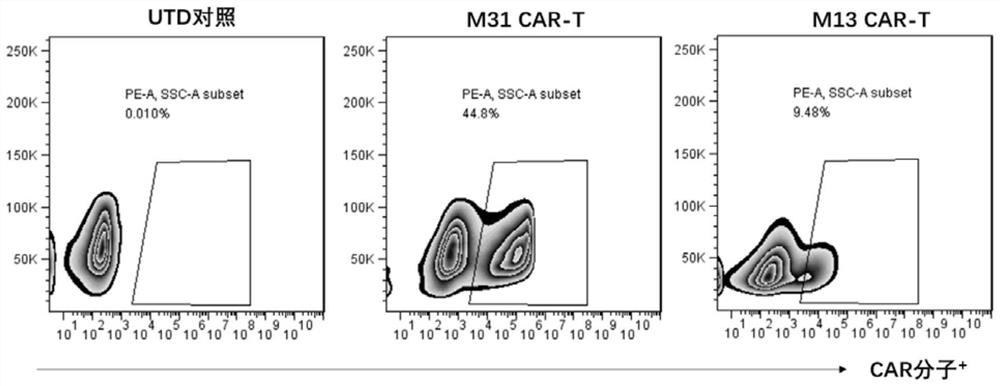
[0055] In a 48-well flat-bottomed cell culture plate (containing 1×10 6 pre-activated peripheral blood mononuclear cells), adding the packaged and concentrated lentiviral vectors (LV-M31-CAR and LV-M13-CAR) (MOI=5~10) in Example 2, adding pro-infection reagent protamine sulfate Protein 10μg / mL, 1000×g, centrifuge at 32°C for 90 minutes, add 1×10 6 Immunomagnetic beads pre-coated with αCD3 / αCD28 antibody were cultured overnight. The next day, replace the culture medium with fresh T cell growth medium to continue culturing. Add fresh T cell growth medium every 2-3 days, and adjust the cell density to 0.5-2×10 6 cells. From 6 to 7 days after infection, remove the immunomagnetic beads of activated T cells, continue to culture and expand T cells modified with bispecific chimeric receptors (M31-CAR or M13-CAR), and wait for the cells to rest (remove Immunization of magnetic beads 6-7 days) before using flow cytometry to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com