Phenothiazine coumarin-based pyridine salt compound as well as preparation and application thereof
A salt compound, coumarin technology, applied in chemical instruments and methods, organic chemistry, instruments, etc., can solve the problems of low detection limit, poor water solubility, low sensitivity of HOCl fluorescent probe, etc., and achieve good biocompatibility Sexuality, the effect of improving water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A phenothiazine coumarinyl pyridinium salt compound, the preparation process of which is as follows:
[0038] (1) Synthesis of Intermediate 1
[0039]
[0040] Iodoethane (3.9g, 25mmol) and 2-methoxyphenothiazine (2.29g, 10mmol) were dissolved in 20mL of dimethyl sulfoxide, then NaOH (0.8g, 20mmol) was added, and the temperature was raised to 65°C for 15 Hour. After the reaction was completed, cool to room temperature, add 300mL of water, add dichloromethane (50mL×2) for extraction, wash with saturated brine (50mL×2), dry over anhydrous sodium sulfate, then remove the solvent under reduced pressure, and use the obtained residue with Separation on a 200-300 mesh silica gel column gave a white solid (2.3 g, 89.5%).
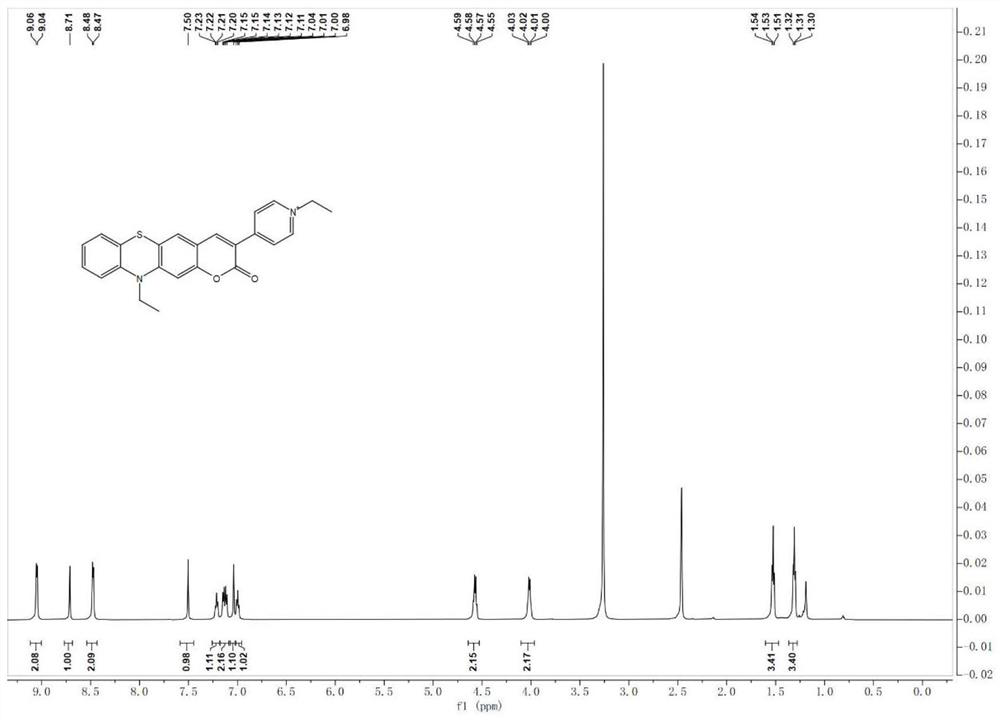
[0041] 1 H NMR (600MHz, DMSO-d 6 )δ7.14 (ddd, J=8.4, 7.2, 1.4Hz, 1H), 7.08 (dd, J=7.6, 1.7Hz, 1H), 7.02–6.94 (m, 2H), 6.92–6.86 (m, 1H) ,6.51(d,J=7.6Hz,2H),3.87(q,J=6.9Hz,2H),3.70(s,3H),1.25(t,J=6.9Hz,3H).
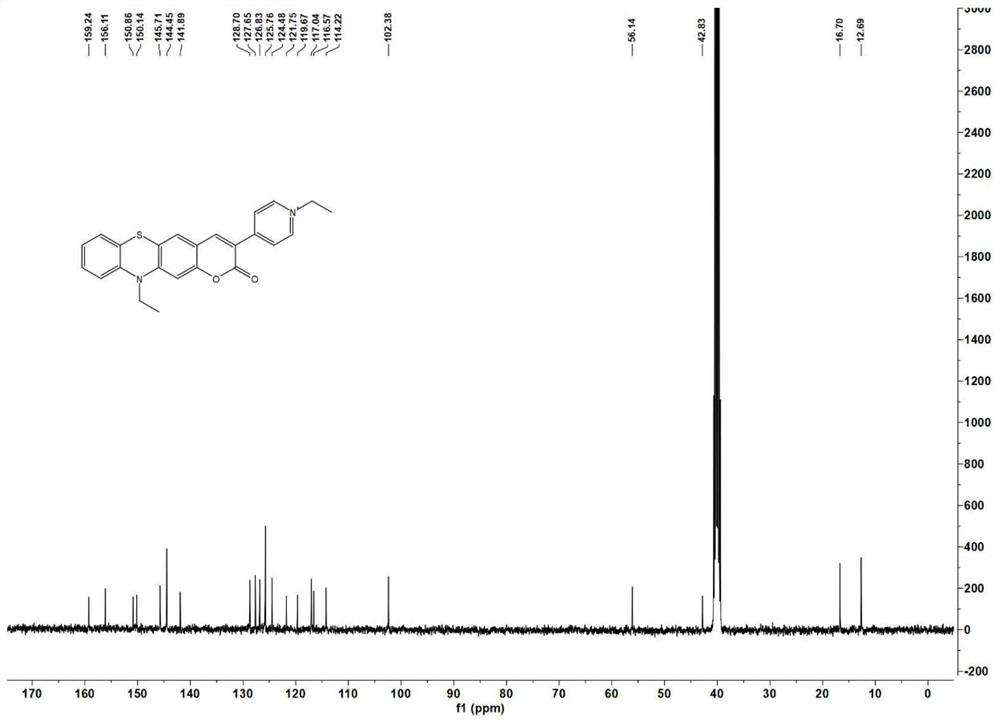
[0042] (2) Synthesis of compound 5
[004...
experiment example 1
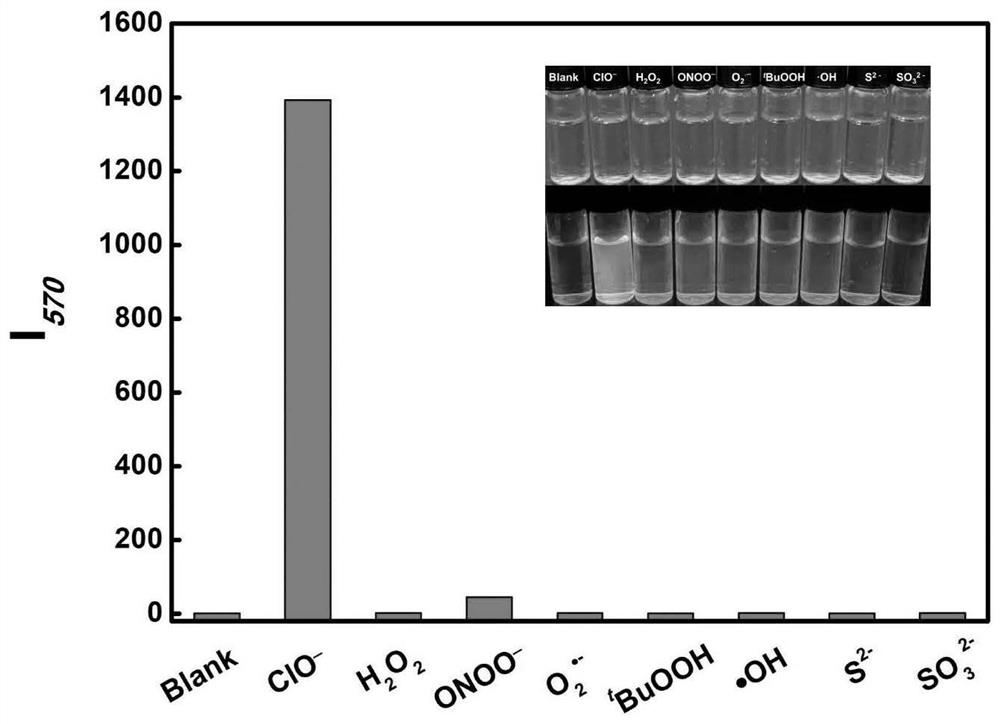
[0061] The selectivity test of experimental example 1 to HOCl
[0062] Prepare the test solution of compound 5 (PBS:EtOH=8:2, V / V, 10μmol / L), take 3mL and transfer it to a quartz cuvette, add different types of active substances, stir slightly for 10s, and test its fluorescence ll.
[0063] Such as image 3 As shown, it is the selectivity test result of compound 5 in Example 1 of the present invention to HOCl, the excitation wavelength is 420nm, image 3 The illustration in is the white light diagram and fluorescence diagram of the compound 5 solution under the action of various active substances, the ordinate I 570 Indicates the emission intensity of compound 5 at 570 nm. The results showed that compound 5 had excellent selectivity to HOCl.
experiment example 2
[0064] Experimental Example 2 Fluorescence intensity test of different concentrations of HOCl
[0065] Prepare the test solution of compound 5 (PBS:EtOH=8:2, V / V, 10μmol / L), take 3mL and transfer it to a quartz cuvette, gradually add HOCl solution dropwise, stir gently for 10s, and test its fluorescence emission spectrum .
[0066] Such as Figure 4 Shown is the fluorescence titration diagram of compound 5 in Example 1 of the present invention against HOCl, and the excitation wavelength is 420nm. Such as Figure 5 Shown is the fluorescence response of compound 5 in Example 1 of the present invention to a low concentration of HOCl, from which the detection limit of compound 5 to HOCl is calculated to be 970pM. The results showed that the detection sensitivity of compound 5 to HOCl was very high.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com