Semaglutide main peptide chain and preparation method thereof
A technology of semaglutide and peptide chain, which is applied in the field of semaglutide main peptide chain and its preparation, can solve the problems that cannot be recycled and reused, by-products are not easy to remove, and affect the purity of peptides, etc., so as to improve the coupling efficiency , reduce synthesis cost, improve the effect of purity and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
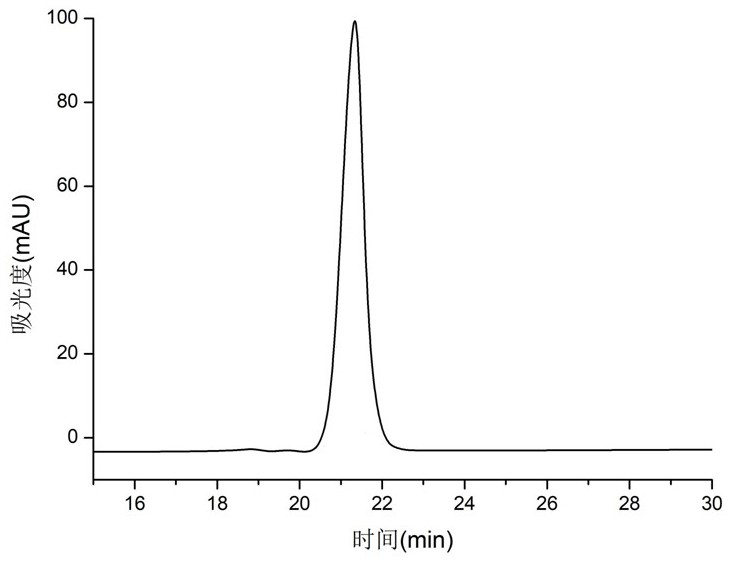
[0097] This embodiment provides a preparation method of a polypeptide condensing agent (synthetic route diagram as shown in figure 1 shown), the methods include:
[0098] i) Add 16.3 g 3,4-dihydro-3-hydroxy-4-oxo-1,2,3-benzotriazine into 100 mL THF and stir to dissolve, add 7.5 g propylene oxide, 0.8 g tetramethyl Ammonium hydroxide was stirred and reacted at 60°C for 4 h, and the product was washed with methanol to obtain product A;
[0099] ii) Dissolve 24.3 g of 2-chloro-1-(2-vinyl-1-piperidinyl)ethanone in 100 mL of DCM, add product A, add 2 g of triethylamine under stirring, continue to Stir the reaction for 4 h, filter, wash the filter cake with methanol, collect the filter cake and dry it to obtain the polypeptide condensing agent.
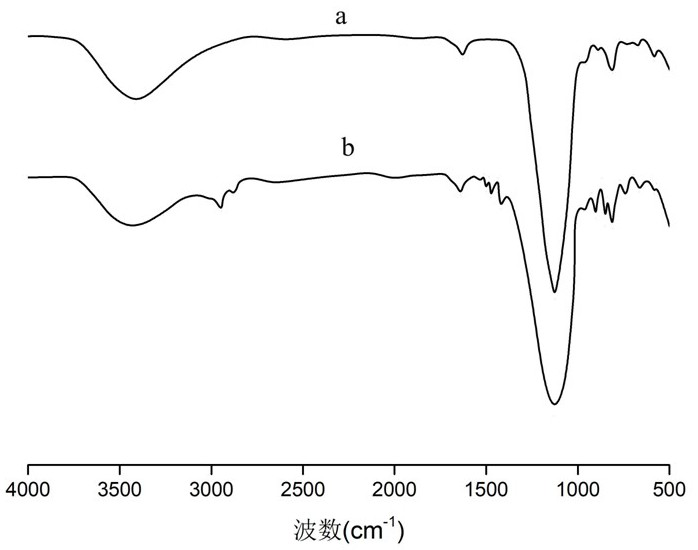
[0100] The obtained polypeptide condensing agent was subjected to the H NMR spectrum test. After the test, the H NMR spectrum was observed. It can be seen that 7.40-8.05 ppm is the chemical shift of the hydrogen on the benzene ring, and 3...
Embodiment 2
[0102] This embodiment provides a preparation method of modified silica gel chromatographic packing, the method comprising:
[0103] Soak C18 silica gel in 0.1 mol / L hydrochloric acid aqueous solution for 3.5 h, then wash with deionized water until neutral, and finally wash with acetone and dry to obtain activated silica gel; dissolve 1 g of activated silica gel in 10 mL of toluene, add 1.4 g2-(3,4-epoxycyclohexyl)methyldiethoxysilane reacted overnight at 90°C, washed with hot water, and dried to obtain epoxidized modified silica gel; 1 g epoxidized The modified silica gel was dissolved in 10 mL of toluene, 0.3 g of 1-pyridin-2-yl-1,4-butanediamine was added at 90°C, stirred and reacted overnight, the product was washed with hot water, and dried to obtain the modified silica gel chromatography packing .
Embodiment 3
[0105] This embodiment provides a method for preparing the main peptide chain of semaglutide, the method comprising:
[0106] Step 1. Preparation of Fmoc-Gly-resin:
[0107]Add 10 g Wang resin (0.5 mmol / g) to 50 mL DCM to swell for 1 h, drain the solvent; add 15 g Fmoc-Gly-OH to 200 mL DCM and stir to dissolve, cool down to below 10°C, add 11.6 g Condensation coupling system, stirred and activated for 20 min, then added to the resin and stirred for 5 h; after the reaction, the solvent was drained, and 1.2 g of acetic anhydride, 1.13 mL of 4-aminopyridine, and 100 mL of DCM were added to capping for 1.5 h, the product DCM was alternately washed with methanol, dried in vacuo to obtain Fmoc-Gly-resin; the degree of substitution was 0.38 mmol / g detected by UV spectrophotometer;
[0108] Step 2, preparing the first polypeptide resin:
[0109] Add the Fmoc-Gly-resin obtained in step 1 to 100 mL DCM to swell for 40 min, drain the solution, add 100 mL, 20 vol% piperidine / DCM solutio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com