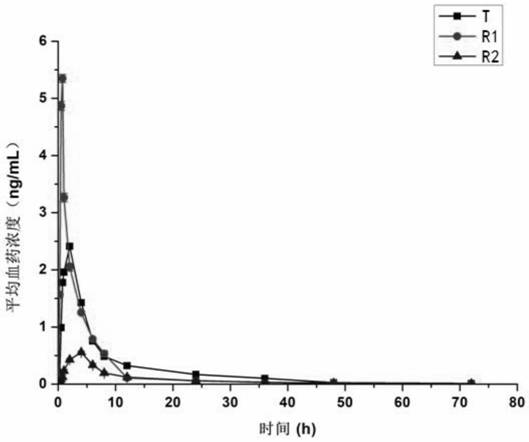
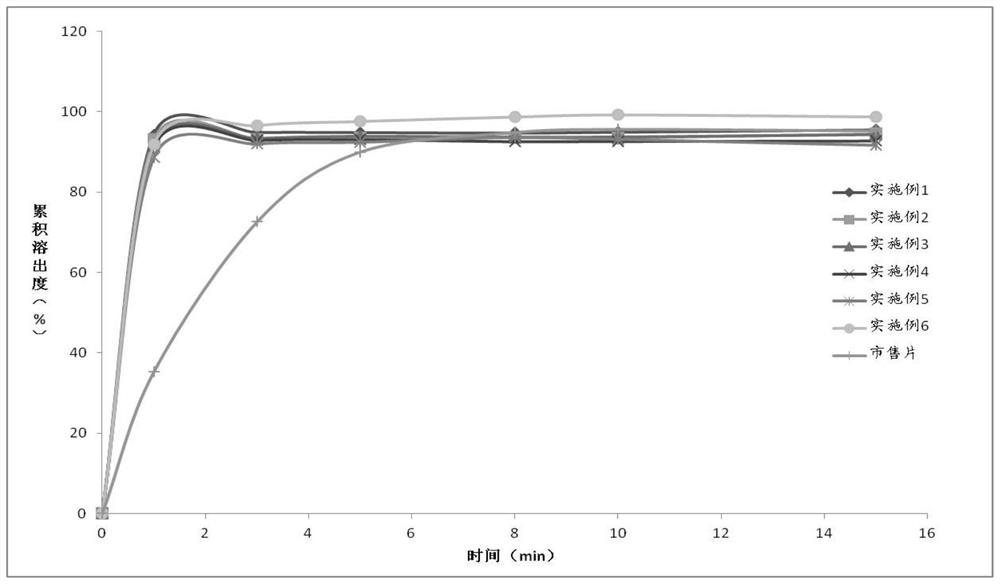
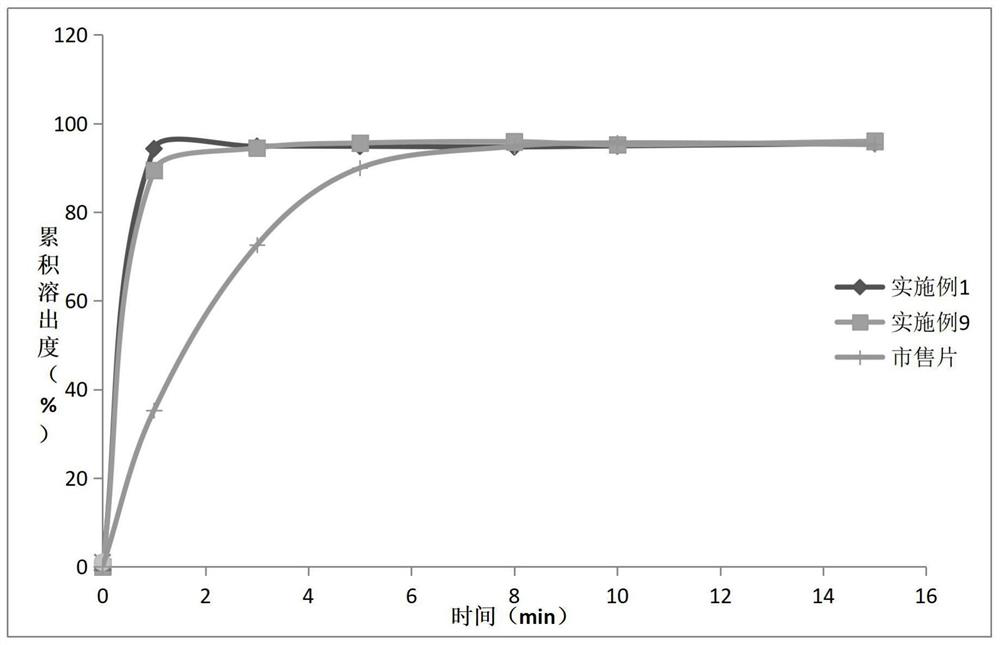
Novel preparation of neostigmine and derivatives thereof
A technology of neostigmine and its derivatives, which is applied in the field of new formulations of neostigmine and its derivatives, can solve unsolved clinical compliance problems and other problems, and achieve the effects of avoiding the first-pass effect, rapid dissolution, and precise dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-6
[0036] The component composition of embodiment 1-6 is as shown in table 1 below:
[0037] Table 1 is the composition table of embodiment 1-6
[0038]
[0039] The preparation steps of the preparation method of embodiment 1 to embodiment 6 are basically the same, and the main difference is that the prescription is different, specifically as follows:
[0040] S1, according to the proportion of the above components, add the prescribed amount of neostigmine bromide, mannitol, sodium carboxymethyl starch, pH regulator sodium carbonate, citric acid, and sodium bicarbonate to the three-dimensional motion mixer, and set the mixing frequency 10Hz, mixing for 20min;
[0041] S2, adding the prescribed amount of magnesium stearate to the mixed powder of S1, setting the mixing frequency to 10 Hz, and mixing for 5 minutes;
[0042] S3, compressing the mixed mixed powder into tablets with a high-speed rotary tablet press equipped with a 6mm circular mold to obtain sublingual bromostigmi...
Embodiment 7-9
[0060] The component composition of embodiment 7-9 is as shown in table 1 below:
[0061] Table 4 is the composition table of embodiment 7-9
[0062]
[0063] The preparation steps of the preparation method of embodiment 7 to embodiment 9 are basically the same, and the main difference is that the prescription is different, specifically as follows:
[0064] Disperse the prescribed amount of hypromellose in purified water at 90°C, stir and dissolve to obtain a colorless and transparent solution, then cool to room temperature and add the prescribed amount of bromoneostigmine, polyethylene glycol, and aspar Tan, edetate disodium, polysorbate, titanium dioxide, sodium carbonate, stirred for 5 minutes; then dispersed with a high-speed disperser for 7 minutes (rotating speed was set at 2000rpm); finally a white milky solution was obtained, and the above white milky solution was ultrasonically sterilized After soaking, it is added to a continuous coating machine for continuous fi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Bronsted acidity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com