Cyclo-trans-4-l-hydroxyprolyl-l-serine-o-amino acid ester and its salts
A technology of hydroxyprolyl and amino acid esters, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
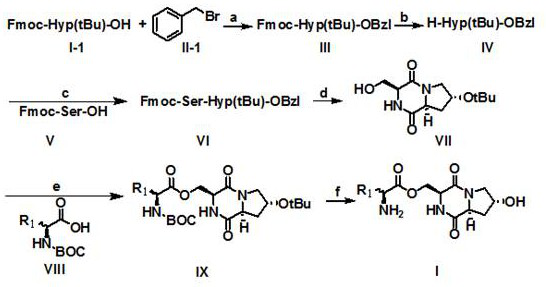
[0052] Step 1: Dissolve compound I, compound II, and sodium carbonate in dichloromethane, fill with nitrogen, react at room temperature for 2 hours, and spin evaporate to obtain compound III.
[0053] Step 2: Dissolve compound III in tetrahydrofuran solution containing diethylamine, react at room temperature for 2 h, evaporate the solvent under reduced pressure, dilute the residue with dichloromethane, wash with 5% phosphoric acid aqueous solution, aqueous solution, and saturated saline successively, and extract Filter, and the filtrate was mixed with silica gel, subjected to silica gel column chromatography, dichloromethane and methanol (100:1-1:100) gradient elution, the column chromatography was collected and concentrated to obtain compound IV.
[0054] The third step: compound IV, compound V, 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDCI), 1-hydroxybenzotriazole (HOBT) were dissolved in In dichloromethane, fill with nitrogen, react at room temperature fo...
Embodiment 2
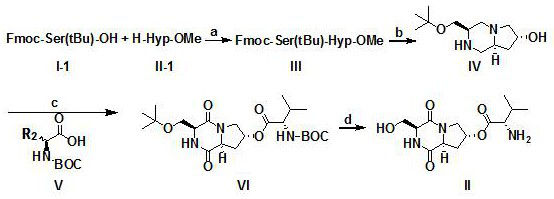
[0059] Step 1: Dissolve compound I, compound II, and sodium carbonate in dichloromethane, fill with nitrogen, react at room temperature for 2 hours, and spin evaporate to obtain compound III.
[0060] Step 2: Dissolve compound III in tetrahydrofuran solution containing diethylamine, react at room temperature for 2 hours, evaporate the solvent under reduced pressure, dilute the residue with dichloromethane, wash with 5% phosphoric acid aqueous solution, aqueous solution, and saturated saline successively, and extract Filter, and the filtrate was mixed with silica gel, subjected to silica gel column chromatography, dichloromethane and methanol (100:1-1:100) gradient elution, the column chromatography was collected and concentrated to obtain compound IV.
[0061] The third step: compound IV, compound V, 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDCI), 1-hydroxybenzotriazole (HOBT) were dissolved in Dichloromethane was reacted at room temperature for 2 h, and the...
Embodiment 3
[0066] Step 1: Dissolve compound I, compound II, and sodium carbonate in dichloromethane, fill with nitrogen, react at room temperature for 2 hours, and spin evaporate to obtain compound III.
[0067] Step 2: Dissolve compound III in tetrahydrofuran solution containing diethylamine, react at room temperature for 2 hours, evaporate the solvent under reduced pressure, dilute the residue with dichloromethane, wash with 5% phosphoric acid aqueous solution, aqueous solution, and saturated saline successively, and extract Filter, and the filtrate was mixed with silica gel, subjected to silica gel column chromatography, dichloromethane and methanol (100:1-1:100) gradient elution, the column chromatography was collected and concentrated to obtain compound IV.
[0068] The third step: compound IV, compound V, 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDCI), 1-hydroxybenzotriazole (HOBT) were dissolved in In dichloromethane, fill with nitrogen, react at room temperatur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com