Heteroaromatic compound, intermediate, composition and application
A compound and heteroaryl technology, applied in the field of heteroaromatic compounds, can solve problems such as deficiencies, and achieve the effect of high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
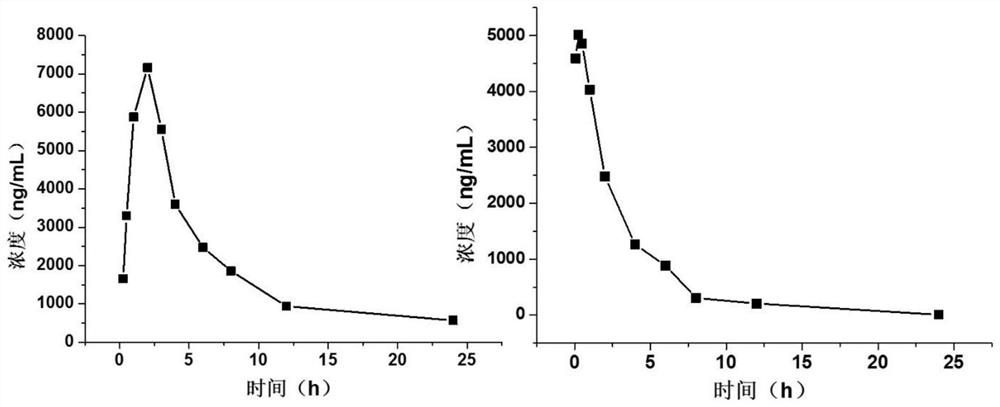
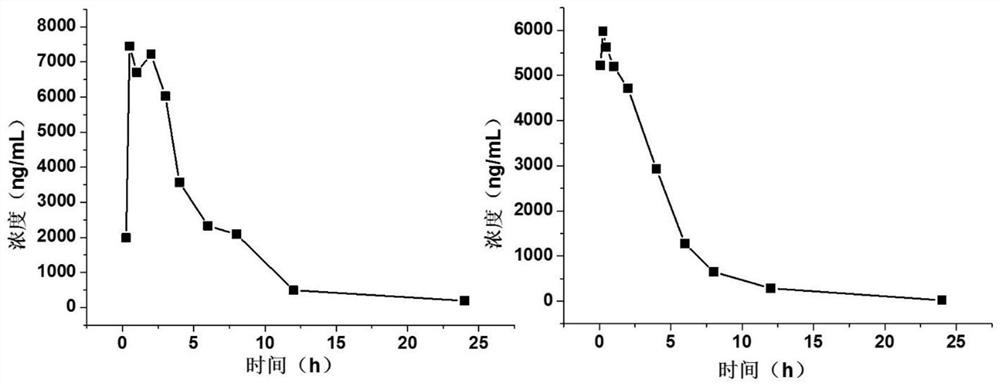
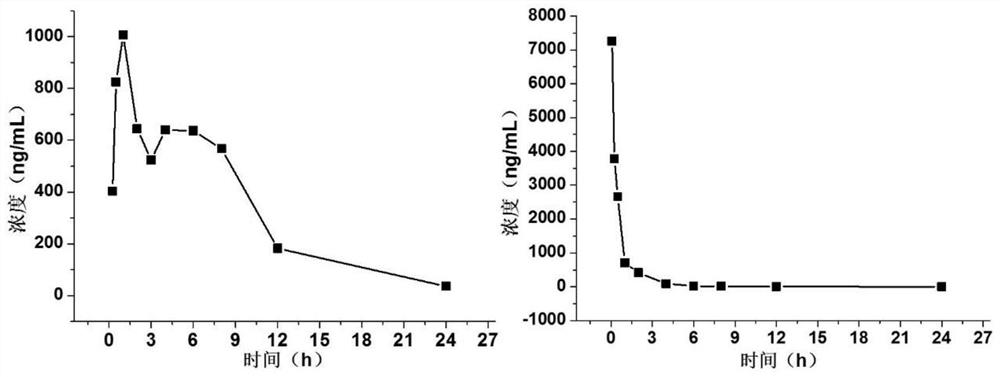
Image
Examples
Embodiment 1
[0091] Example 1. Intermediate compound 3-(2-amino-6-ethynylpyrimidin-4-yl)-2-methylbenzonitrile (8)
[0092]
[0093] Step 1.1. Synthesis of 2-methyl-3-cyanophenylboronic acid pinacol ester (3)
[0094] Add compound 1 (10.00g, 51mmol) into a 500mL three-neck flask, add 100mL 1,4-dioxane, and stir to dissolve. Under nitrogen protection, compound 2 (15.54g, 61mmol), potassium acetate (10.01g, 102mmol) and Pd(dppf)Cl were added sequentially 2 (1.12 g, 1.53 mmol). The reaction was stirred under reflux for 5h, and the temperature was lowered to 25°C. Suction filtration, and the filter cake was washed with 50 mL of ethyl acetate. Add H to the filtrate 2 50 mL each of O and ethyl acetate, stirred for 5 min, separated, the aqueous phase was extracted with ethyl acetate (30 mL × 3), the ethyl acetate phase was combined, and then washed with 30 mL of saturated brine, anhydrous Na 2 SO 4 Dry for 5 minutes, filter with suction, and concentrate under reduced pressure to obtain a ...
Embodiment 2
[0101] Example 2 Compound 3-(2-amino-6-(1-(quinolin-2-ylmethyl)-1H-1,2,3-triazol-4-yl)pyrimidin-4-yl)-2 - Preparation of methylbenzonitrile (11) (BS001)
[0102] The synthetic route is as follows:
[0103]
[0104] Step 2.1: Synthesis of 2-(azidomethyl)quinoline (10)
[0105] At 25°C, under nitrogen protection, compound 9 (1.00 g, 4.50 mmol) was added into a 50 mL three-neck round bottom flask. Add 10 mL of anhydrous tetrahydrofuran and stir to dissolve. Cool the system down to -10°C, add TMSN dropwise 3 (1.04g, 9.02mmol) and DIPEA (1.16g, 8.98mmol). After the dropwise addition, the temperature of the system was raised to 25° C., and the reaction was stirred for 24 hours. The insoluble matter was removed by suction filtration, the solvent was removed by concentration under reduced pressure, and silica gel column chromatography (n-hexane: ethyl acetate = 10:1) gave 0.515 g of a light yellow liquid (compound 10, yield 62%). MS(ESI)m / z:185.1[M+H] + , 1 H NMR (400MHz, C...
Embodiment 3
[0108] Example 3 3-(2-amino-6-(1-((8-aminoquinolin-2-yl)methyl)-1H-1,2,3-triazol-4-yl)pyrimidine-4- The preparation of -2-methylbenzonitrile hydrochloride (16) (BS002)
[0109] The synthetic route is as follows:
[0110]
[0111] Step 3.1: Synthesis of 2-(bromomethyl)-8-nitroquinoline (13)
[0112] Compound 12 (1.42 g, 10 mmol) was added into a 100 mL three-necked flask, and then 30 mL of carbon tetrachloride was added. N-bromosuccinimide (2.67 g, 15 mmol) and 0.2 g of benzoyl peroxide were added sequentially under stirring at 25°C. Heated to reflux and reacted under reflux for 14 hours. The reaction solution was suction filtered to remove insoluble matter, and concentrated under reduced pressure to obtain 2.1 g of a yellow solid, which was subjected to silica gel column chromatography (n-hexane: ethyl acetate = 3:1) to obtain 1.69 g of a white solid (compound 13, yield 63.5%) . MS(ESI)m / z:222.0,224.0[M+H] + , 1 H NMR (400MHz, CDCl 3 ): δ8.17(d, J=8.5Hz, 1H), 8.08(d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com