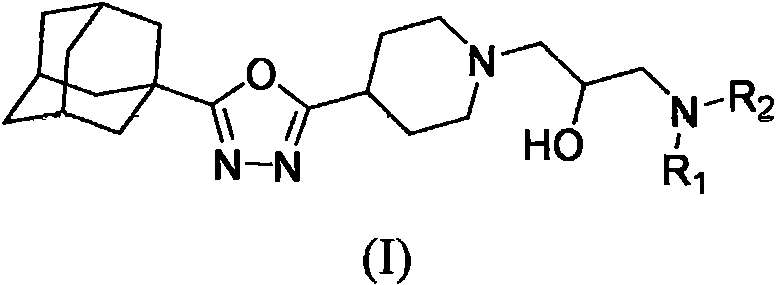
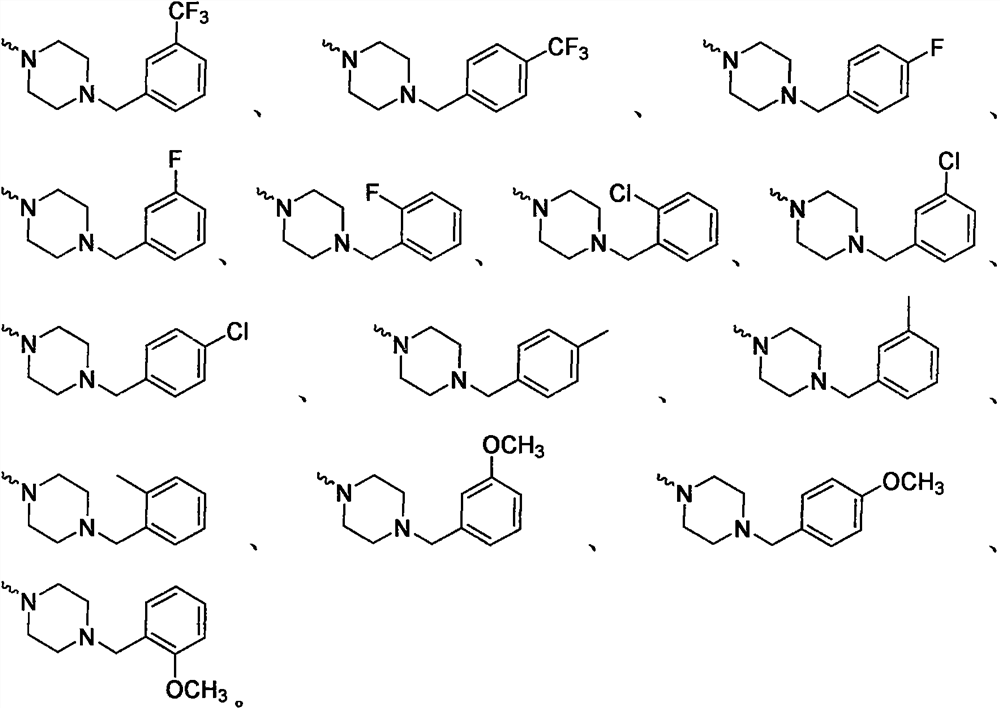
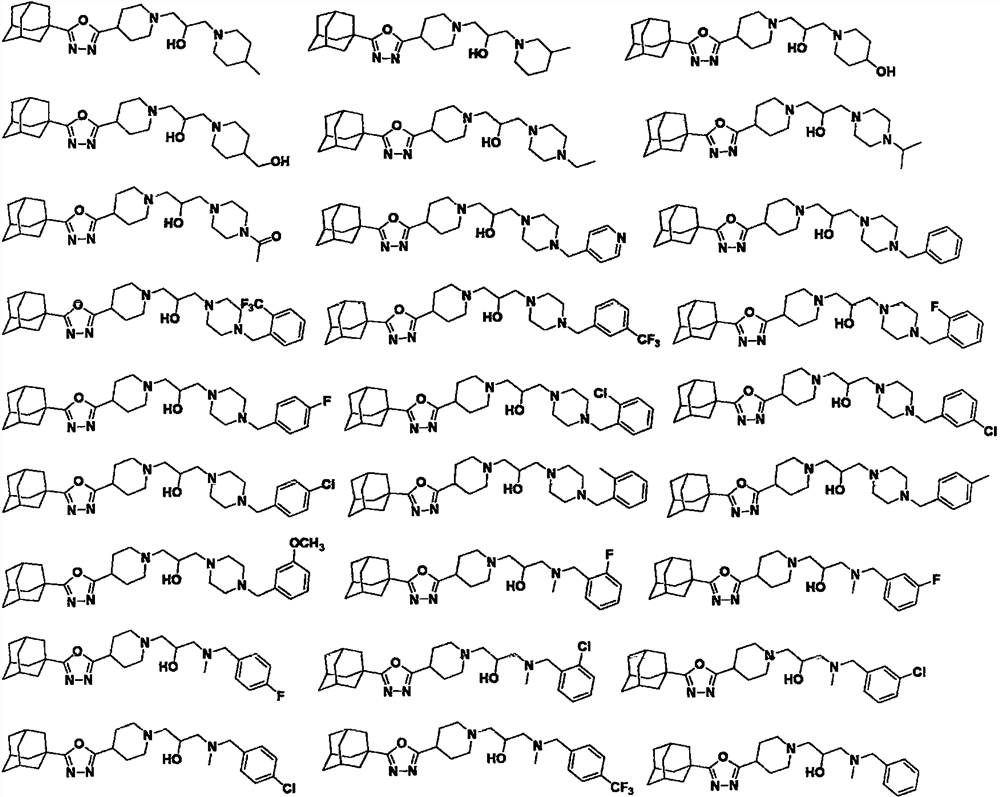
Adamantyl-containing oxadiazole compound as well as preparation method and application thereof
A technology of adamantyl oxadiazoles and compounds, applied in the field of adamantyl oxadiazoles and their preparation, capable of solving problems such as leaf wilting, plant necrosis, and economic losses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: the preparation of intermediate 1-adamantyl methyl carboxylate
[0044] Add 1-adamantylcarboxylic acid (110.96mmol) into a 250mL round-bottomed flask containing 100mL of methanol, then add 3mL of concentrated sulfuric acid, and condense and reflux for 12h. After the temperature of the reaction system returned to room temperature, it was extracted with ethyl acetate and water, and the organic phase was collected and dried with anhydrous sodium sulfate, and after precipitation, the colorless oily intermediate 1-adamantylmethyl carboxylate was obtained with a yield of 98.0% . Its NMR data are: 1 H NMR (400MHz, CDCl 3 )δ3.51(s, 3H, O-CH 3 ), 1.87 (s, 3H, adamantaneyl-3, 5, 9), 1.77 (t, J=6.8Hz, 6H, adamantaneyl-4, 6, 8), 1.63-1.52 (m, 6H, adamantaneyl-2, 7 , 10).
Embodiment 2
[0045] Embodiment 2: the preparation of intermediate adamantane-1-carboxyhydrazine
[0046] Add 80 mL of hydrazine hydrate (content 80%) to the intermediate 1-adamantyl formate methyl ester (110.96 mmol) obtained above, and stop the reaction after reflux reaction for one week. After the reactant is cooled in an ice-water mixture, a white solid will precipitate out, and the intermediate 1-adamantylcarbohydrazide is obtained by suction filtration as a white solid with a melting point of 155-156° C. and a yield of 99.0%. Its NMR data are: 1 H NMR (400MHz, CDCl 3 )δ6.95(s, 1H, C-NH-), 2.74(s, 2H, NH- NH 2 -), 2.04 (s, 3H, adamantaneyl-3, 5, 9), 1.94-1.80 (m, 6H, adamantaneyl-4, 6, 8), 1.72 (q, J=12.3Hz, 6H, adamantaneyl-2, 7, 10); 13 C NMR (101MHz, CDCl 3 )δ178.8, 40.2, 39.2, 36.7, 28.2.
Embodiment 3
[0047] Embodiment 3: Preparation of intermediate 2-(1-adamantyl)-5-(4-piperidinyl)-1,3,4-oxadiazole
[0048] Weigh 1-adamantylcarbohydrazide (12.87mmol) and 4-piperidinecarboxylic acid (12.87mmol) into a 50mL round bottom flask, then add 20mL phosphorus oxychloride, react at 80°C for 48h, then stop the reaction. POCl was distilled off under reduced pressure 3Finally, the reactant was dissolved in 100 mL of ethyl acetate, and extracted with water; the aqueous layer collected by extraction was adjusted to pH 10-11 with sodium hydroxide solution, and then extracted with dichloromethane, and the organic phase was dried over anhydrous sodium sulfate, Precipitation, separation and purification by column chromatography gave the intermediate 2-(1-adamantyl)-5-(4-piperidinyl)-1,3,4-oxadiazole as a yellow oil with a yield of 75.7% . 1 H NMR (400MHz, CDCl 3 ( q, J=16.8Hz, 2H, piperidin-H), 2.07(s, 3H, admantane-H), 2.04-1.91(m, 8H, admantane-H & piperidin-H), 1.81-1.70(m, 6H, admant...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com