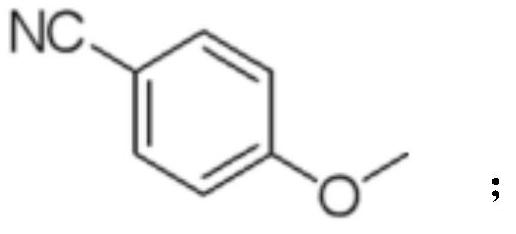
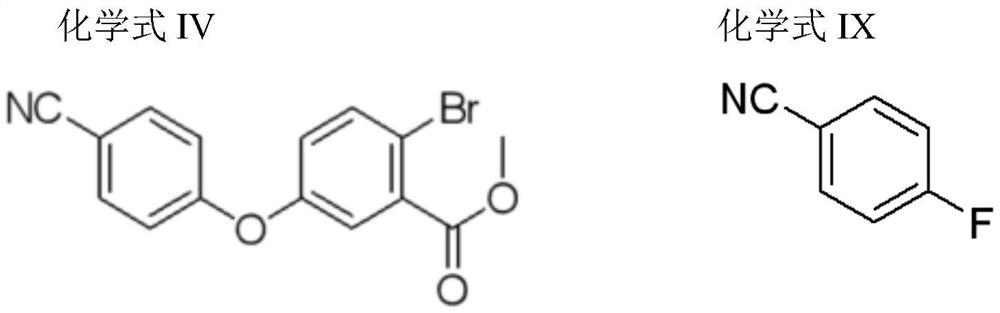
Preparation method of crisaborole and intermediate product thereof
A technology of crisborol and its products, which is applied in the field of preparation of crisborol and its intermediate products, can solve the problems of harsh reaction conditions and unfavorable scale-up production, and achieve short reaction steps, reduced by-products, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] The reaction formula for the preparation of compound III is as follows:
[0066]
[0067] Add compound II (5.0 g, 0.023 mol) and methanol (40 mL) into the three-necked flask, and add 10 drops of concentrated sulfuric acid dropwise. After the addition, the temperature of the reaction system is raised to 60° C., and the reaction is stopped after the completion of the reaction as detected by TLC;
[0068] The reaction solution was concentrated under reduced pressure, 25ml of ethyl acetate and 15ml of water were added to the concentrate, and 15ml of saturated NaHCO was added to the organic layer after liquid separation. 3 After solution washing and liquid separation, the organic layer was washed with 15 ml of saturated brine. After liquid separation, the organic phase was dried with anhydrous sodium sulfate, filtered and concentrated under reduced pressure to obtain a white solid (5.04 g, 96.7% yield); 1 H-NMR (400Hz, DMSO-d 6 )δ (ppm) 3.81 (s, 3H), 6.89 (dd, J = 2.0, 2...
Embodiment 2
[0070] The reaction formula for the preparation of compound III is as follows:
[0071]
[0072] Add compound II (50.0 g, 0.23 mol) and methanol (400 mL) to the three-necked flask, and add 7.5 ml of concentrated sulfuric acid dropwise. After the addition, the temperature of the reaction system is raised to 60° C., and the reaction is stopped after TLC detects that the reaction is complete;
[0073] Concentrate the reaction solution under reduced pressure, add 250ml ethyl acetate and 150ml water to the concentrate, add 150ml saturated NaHCO to the organic layer after liquid separation 3 After solution washing and separation, the organic layer was washed with 150 ml of saturated brine. After separation, the organic phase was dried with anhydrous sodium sulfate, filtered and concentrated under reduced pressure to obtain a white solid (51.3 g, 96.4% yield).
Embodiment 3
[0075] The reaction formula of compound IV preparation is as follows:
[0076]
[0077] Add compound III (4.9g, 0.021mol), N,N-dimethylformamide (15mL), potassium carbonate (5.86g), p-fluorobenzonitrile (5.14g) into the three-necked flask, and the temperature of the reaction system rises after the addition is complete To 110°C, stop the reaction after TLC detects that the reaction is complete;
[0078] Filtrate, add the filtrate to ml water to quench, filter to obtain off-white solid (6.52g, 95.6% yield); 1 H-NMR (400Hz, DMSO-d 6 )δ (ppm) 3.82 (s, 3H), 7.18 (d, J = 8.4Hz, 2H), 7.26 (dd, J = 2.8, 2.4Hz, 1H), 7.49 (d, J = 2.4Hz, 1H), 7.79 (d, J=8.0Hz, 1H), 7.86 (d, J=8.8Hz, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com