Preparation method of oleophylic Fe-based suspended bed hydrocracking catalyst
A catalyst and lipophilic technology, applied in the field of preparation of lipophilic Fe-based suspended bed hydrocracking catalysts, can solve the problems of unpublished preparation, harsh preparation conditions, etc., achieve good application prospects, improve yield and crude oil Conversion rate, the effect of cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The steps for preparing mesoporous iron oxide are the same as those in the comparative example.
[0040] Dissolve 1.0 g of sodium stearate in 40 mL of n-hexane, stir at room temperature for 30 min, take 2.0 g of mesoporous iron oxide and disperse it in the organic solution, stir in water at 70 °C for 2 h, and sonicate for 20 min; transfer the mixture to 100 mL crystallization kettle, heat reaction at 100°C for 24 h; naturally cool to room temperature, centrifuge and wash with absolute ethanol to obtain a reddish-brown solid, and dry the solid at 100°C for 12 hours to prepare lipophilic mesoporous iron oxide .
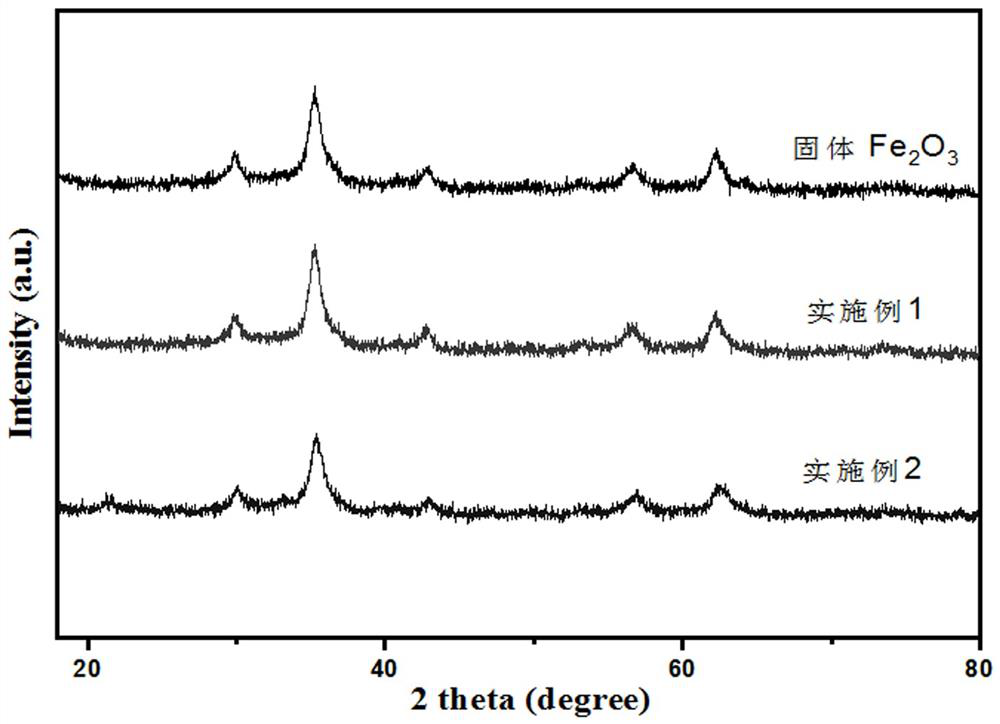
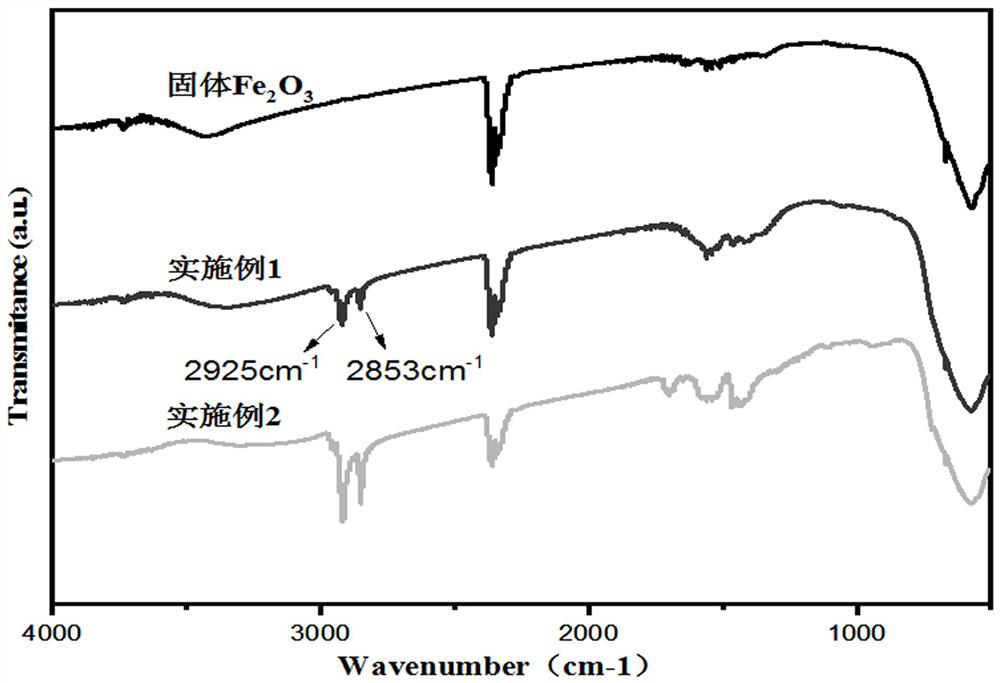
[0041] The lipophilic mesoporous iron oxide XRD spectrogram of present example gained is as follows figure 1 As shown, the dispersibility of lipophilic mesoporous iron oxide obtained in this example in gasoline is as follows figure 2 As shown, the Fourier transform infrared spectrum of the lipophilic mesoporous iron oxide obtained in this example is as follows...
Embodiment 2
[0044] The steps for preparing mesoporous iron oxide are the same as those in the comparative example.
[0045] Dissolve 1.0 g of stearic acid in 40 mL of deionized water, stir at room temperature for 30 min, take 2.0 g of mesoporous iron oxide and disperse it in the organic solution, stir in water at 70 °C for 2 h, and sonicate for 20 min; transfer the mixture to 100 mL In a crystallization kettle, react in an oven at 120°C for 24 h; naturally cool to room temperature, centrifuge and wash with absolute ethanol to obtain a reddish-brown solid, and dry the solid at 110°C for 12 hours to obtain lipophilic mesoporous iron oxide .
[0046] The lipophilic mesoporous iron oxide XRD spectrogram of present example gained is as follows figure 1 As shown, the dispersibility of lipophilic mesoporous iron oxide obtained in this example in gasoline is as follows figure 2 As shown, the Fourier transform infrared spectrum of the lipophilic mesoporous iron oxide obtained in this example is...
Embodiment 3
[0049] The steps for preparing mesoporous iron oxide are the same as those in the comparative example.
[0050] 2.0 g of stearic acid was dissolved in 40 mL of toluene, stirred at room temperature for 30 min, 4.0 g of mesoporous iron oxide was dispersed in the organic solution, stirred in water at 70 °C for 2 h, and ultrasonically treated for 20 min; the mixture was transferred to 100 mL crystal React in an oven at 120°C for 24 h; naturally cool to room temperature, centrifuge and wash with absolute ethanol to obtain a reddish-brown solid, dry the solid at 110°C for 12 hours, and sample and package.
[0051] The experimental conditions of the catalyst suspension bed hydrocracking reaction are the same as those of the comparative example, and the reaction results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com