Application of oridonin and/or prodrug thereof in preparation of medicine for inhibiting SARS-CoV-2
A technology of oridonin and sars-cov-2, which is applied in the application field of medicine to achieve the effect of reducing activity and inhibiting RNA replication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
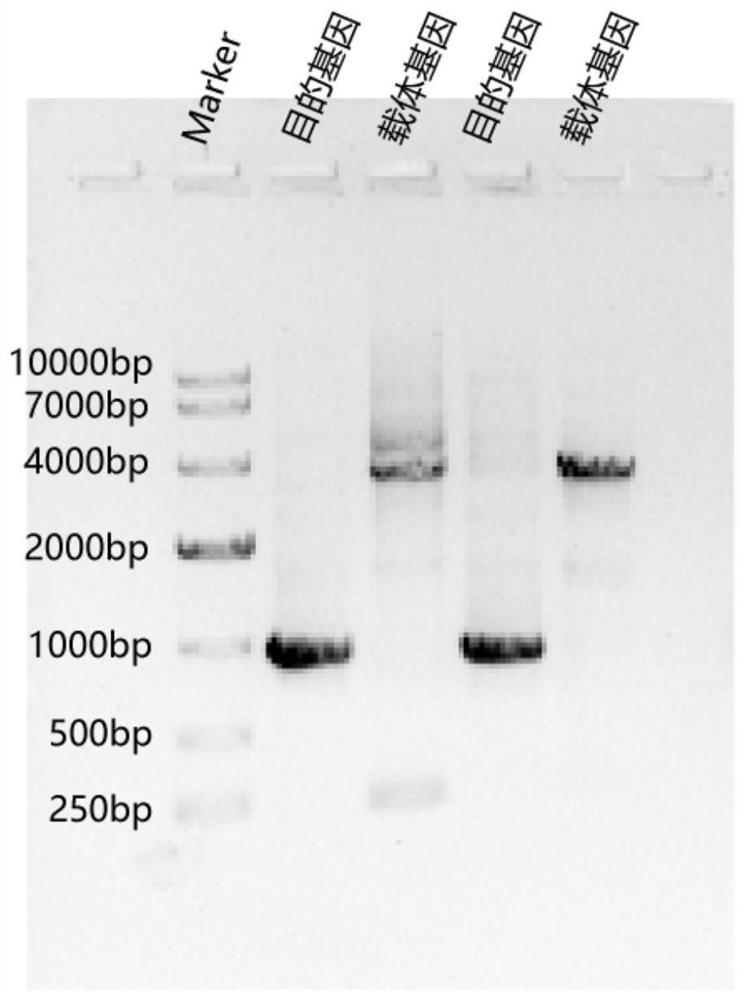
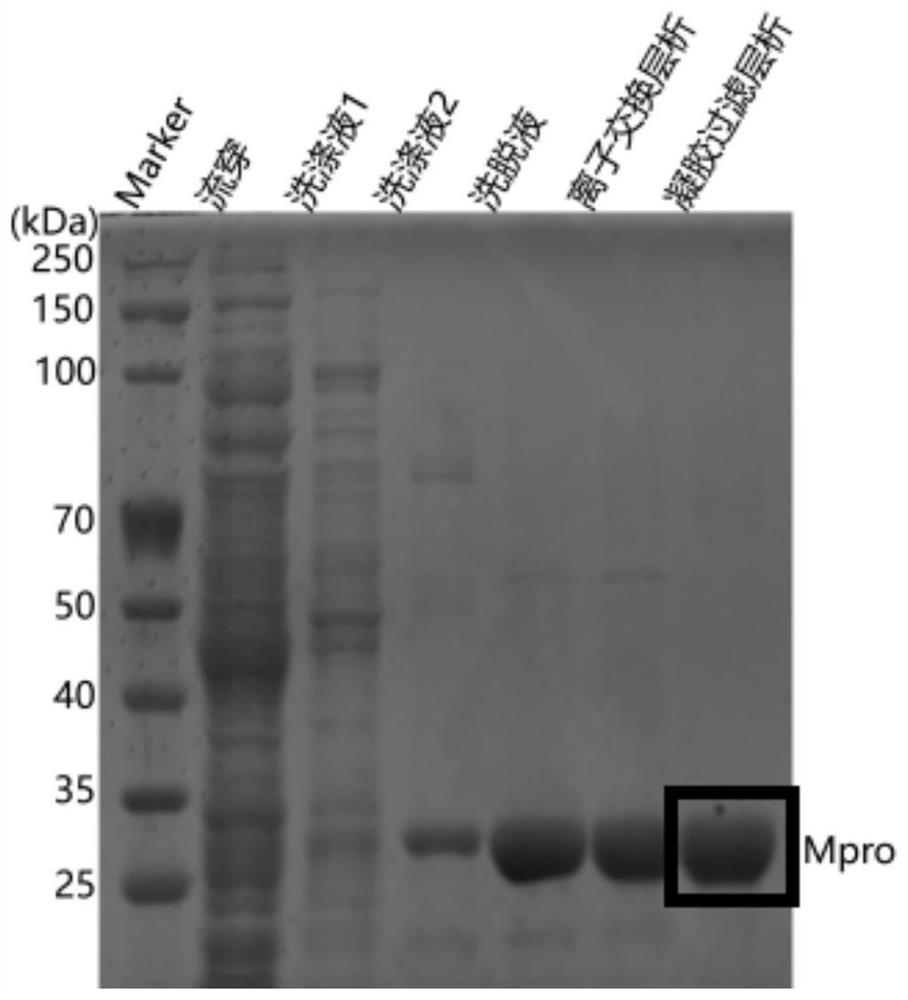
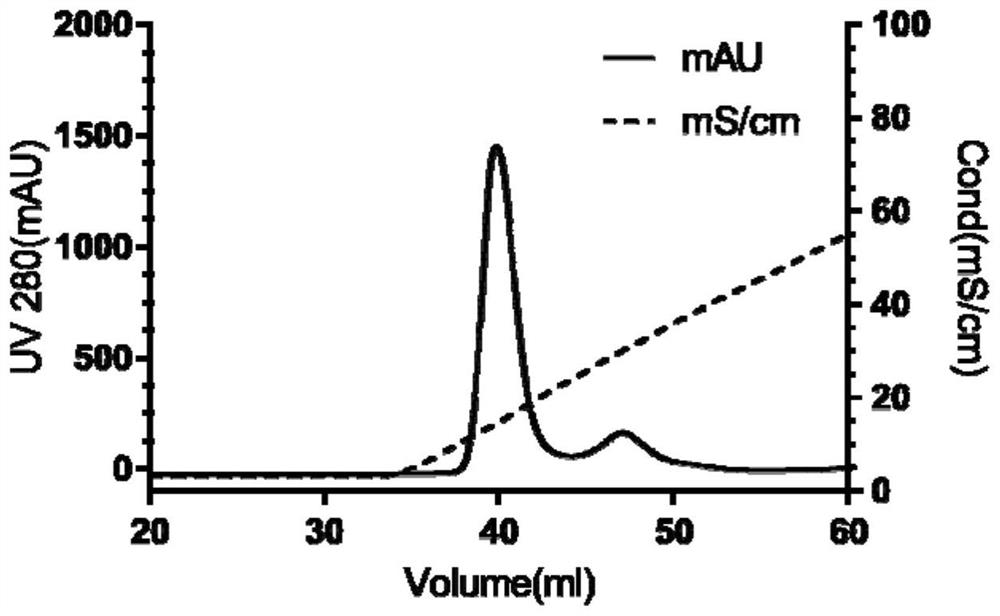
[0037] Construction and protein expression and purification of Mpro protease fusion plasmid in Example 1 SARS-CoV-2
[0038] 1. Codon optimization:
[0039] Select the Mpro protease gene in SARS-CoV-2, with a full length of 918bp; analyze its gene sequence according to the general genetic code table and the codon preference of E. The codons preferred by Bacillus replace the codons with different preferences in the Mpro protease gene, and the codon-optimized Mpro protease gene sequence is designed and chemically synthesized. The coding sequence is shown in SEQ ID NO.1.
[0040] 2. Primer design:
[0041] Design primers F1 and R1 to amplify the codon-optimized Mpro protease gene, design primers F2 and R2 to amplify pET-20b: primer F1 is shown in SEQ ID NO.2, primer F2 is shown in SEQ ID NO.3, R1 is the reverse complementary sequence of F2, and R2 is the reverse complementary sequence of F1.
[0042] Primer sequence Primer F1 (SEQ ID NO.2) 5′-GATATACATATGAGC...
Embodiment 2
[0058] Example 2 Effect of Rubescensine A on Fluorescent Thermal Shift of Mpro Protease in SARS-CoV-2
[0059] Mpro protease was diluted to a final concentration of 0.2 mg / mL with reaction buffer (20 mM Tris-HCl pH8.0, 150 mM NaCl, 1 mM DTT), and 100 μM oridonin and 4×SYPRO orange fluorescent dye (purchased (from Sigma-Aldrich Company), the final reaction volume was 20 μL, the mixture was incubated at 25°C for 10min, and then heated from 25°C to 95°C at a heating rate of 1°C / min; only 0.2mg / mL of Mpro protease and 4× The mixture of SYPRO orange fluorescent dye was the negative control group. Three replicate wells were set up for each experiment, and the experiment was repeated 3 times. Use the StepOnePlus real-time PCR instrument (purchased from Life Technologies) to record the fluorescence intensities of the two groups, and explore the effect of oridonin on the Mpro protease fluorescence heat in SARS-CoV-2 by fluorescence thermal shift analysis (Fluorescence-based Thermal sh...
Embodiment 3
[0060] Example 3 Oridonin A inhibits Mpro protease in SARS-CoV-2 in vitro
[0061] (1) Mpro protease substrate design:
[0062]Known Mpro protease hydrolysis substrate peptide sequence SEQ ID NO.4: -KTSAVLQSGFRKME-(-Lys-Thr-Ser-Ala-Val-Leu-Gln-Ser-Gly-Phe-Arg-Lys-Met-Glu-), The Edans fluorescent group was added to the N-terminal of the peptide chain, and the Dabcyl fluorescent quenching group was added to the C-terminal of the peptide chain to finally synthesize Edans-KTSAVLQSGFRKME-Dabcyl (synthesized by Jill Biochemical (Shanghai) Co., Ltd.).
[0063] (2) Detection of the inhibitory effect of oridonin on Mpro protease by fluorescence resonance energy transfer method:
[0064] Gradient concentrations of oridonin (0μM, 0.12μM, 0.47μM, 1.87μM, 3.75μM, 7.5μM, 15μM, 60μM) were added to the above enzyme activity experiment, and reaction buffer (20mM Tris-HCl pH8.0 , 150mM NaCl, 1mM DTT) to dilute Mpro protease to a final concentration of 0.5μM, and at the same time add 50μM of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com