Method for enhancing synergistic oxidation of sodium sulfite and glucose by using platinum modified titanium dioxide electrode or nickel oxide electrode
A technology of titanium dioxide and sodium sulfite, applied in the field of electrochemical catalysis, can solve the problems of restricting the wide application of electrolyzed water and high power consumption, and achieve the effects of improving the utilization rate of precious metals, reducing production costs, and enhancing practical value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Preparation of platinum-modified titanium dioxide electrode and nickel oxide electrode
[0029] The platinum-modified titanium dioxide electrode of this embodiment is prepared by the following method:
[0030] (1) Weigh 0.03g of P25 high-purity titanium dioxide powder calcined at 450°C, add 3mL of absolute ethanol, put it into an ultrasonic cleaner and stir for 60 minutes, then dip the indium tin oxide ITO electrode in the above colloidal solution for 30 seconds, Then pull it up and dry it in a constant temperature box to make a titanium dioxide / ITO electrode;
[0031] (2) prepare 20mL 0.1mol / L potassium chloride solution, use this solution as supporting electrolyte to prepare 0.01mol / L chloroplatinic acid solution;
[0032] (2) Adopt constant potential method electrodeposition technology, reaction is carried out in three-electrode system, working electrode is the titanium dioxide / ITO electrode that above-mentioned step (1) makes, and counter electrode is tit...
Embodiment 2
[0038] Embodiment 2 Construction of electrocatalytic fuel cell
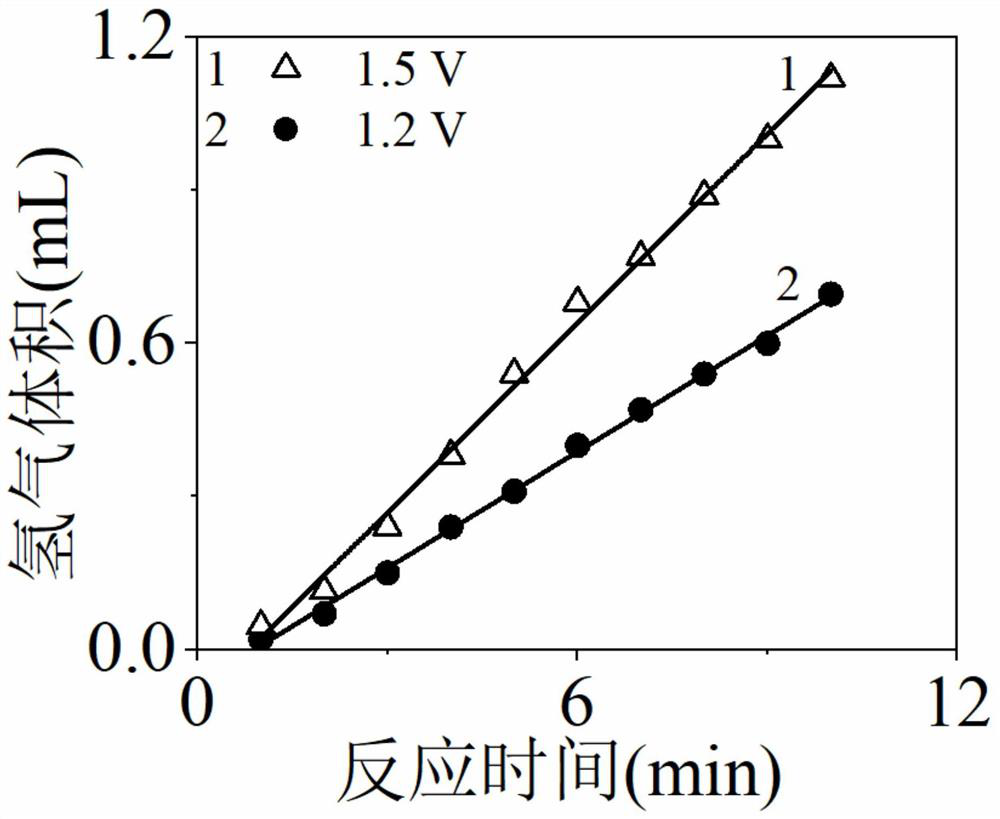
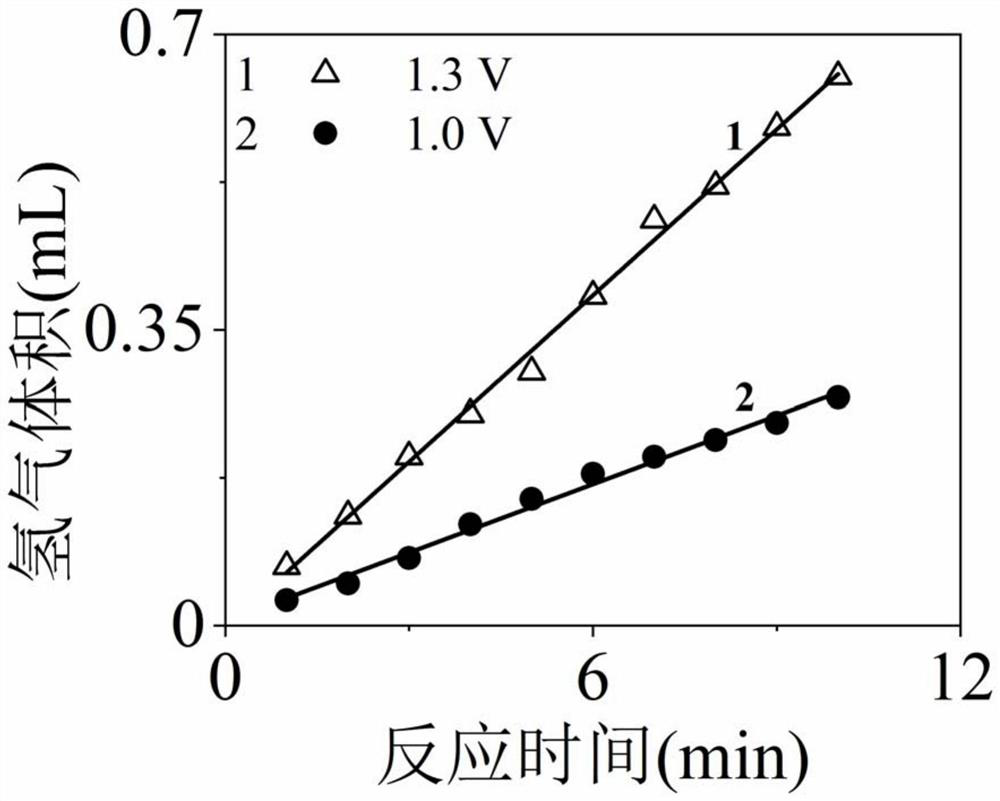
[0039]Using the platinum-modified titanium dioxide electrode or nickel oxide electrode prepared in Example 1 as the anode, the saturated calomel electrode as the reference electrode, and the porous nickel foam electrode as the cathode, a three-electrode electrode was constructed with the anode, cathode, reference electrode and electrochemical workstation. Chemical reaction system, add 0.5mol / L sodium sulfate (as supporting electrolyte solution), 0.1mol / L glucose (as fuel) and 0.1mol / L sodium sulfite (fuel and synergistic oxidation catalyst) to the anode chamber, add 0.5mol / L sodium sulfate solution, the cathode chamber and the anode chamber are connected with Nafion 117 proton exchange membrane to form an electrocatalytic fuel cell; wherein a plastic tube with a scale of 5 milliliters is added to the cathode chamber to cover the cathode electrode, and the closed plastic The nozzle is punched at the bottom of the...
Embodiment 3
[0042] Example 3 Platinum-modified titanium dioxide electrode and nickel oxide electrode enhance the synergistic oxidation of glucose by sodium sulfite
[0043] In order to further illustrate the synergistic oxidation of sodium sulfite to glucose in the electrocatalytic fuel cell constructed in Example 2, the specific steps are as follows:
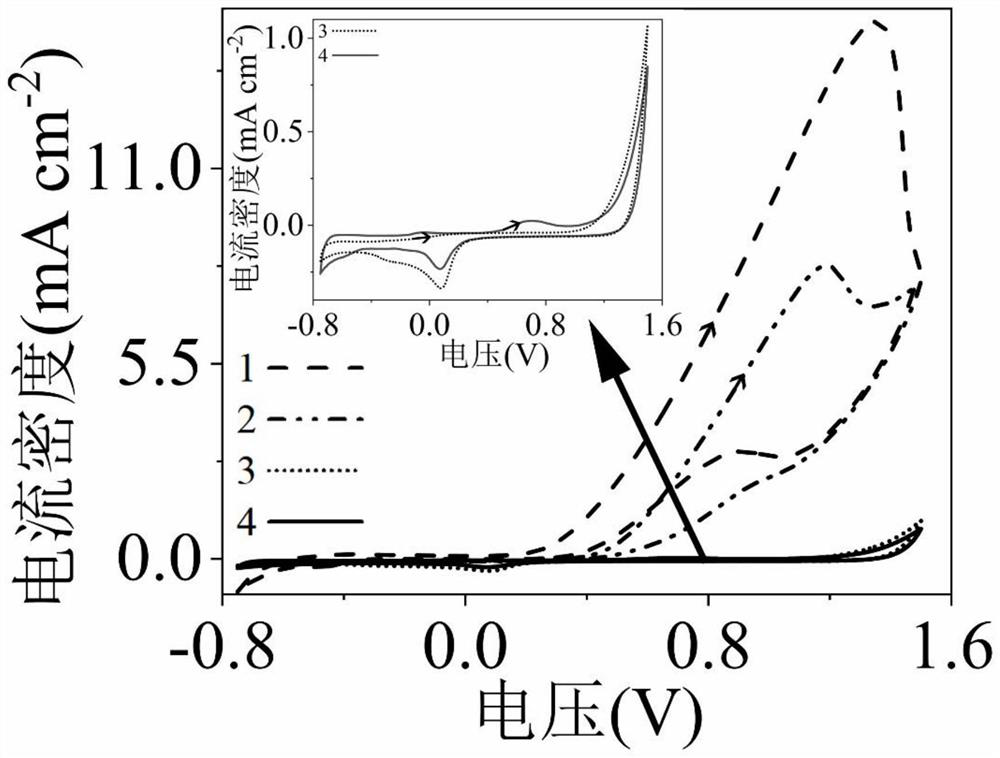
[0044] (1) Using cyclic voltammetry electrochemical measurement technology, in the three-electrode system, the working electrode is a platinum-modified titanium dioxide electrode or a nickel oxide electrode, the counter electrode is a titanium electrode, and the reference electrode is a saturated calomel electrode;
[0045] (2) The following four solutions were filled into the anode chamber to clarify the role of platinum-modified titanium dioxide electrode in enhancing the oxidation of sodium sulfite and the synergistic oxidation of glucose by sodium sulfite. 1 is 0.5mol / L sodium sulfate solution, 0.1mol / L sodium sulfite and 0.1mol / L gluc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com