Primers and probes for detecting SARS-CoV-2 and application of primers and probes
A coronavirus and probe technology, applied in the field of medical detection, can solve the problems of heavy primer design and screening workload, long treatment cycle, high viral load, etc., achieve good primer-probe combination and reagent specificity, and simple result interpretation Ease of understanding and high detection throughput
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0118] Embodiment 1 Novel coronavirus detection method steps
[0119] 1.1 Template preparation
[0120] (1) For the new coronavirus Delta variant, take 200uL directly and use nucleic acid extraction reagents to extract viral RNA on an automatic nucleic acid extraction instrument.
[0121] (2) New coronavirus pseudovirus: adjust the concentration with PBS buffer to obtain 10 4 Copies / mL and 500copies / mL two concentrations, using nucleic acid extraction reagents, extract pseudovirus RNA on a fully automatic nucleic acid extraction instrument.
[0122] (3) Pharyngeal swabs of healthy volunteers and other pathogen-positive specimens or cultures: take 200uL of nucleic acid extraction reagents, and extract nucleic acid from the specimens on an automatic nucleic acid extraction instrument.
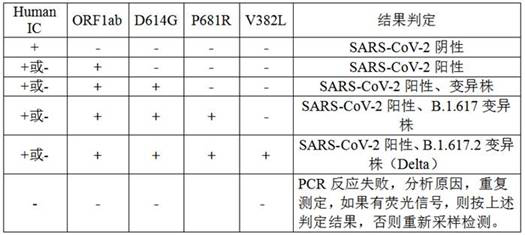
[0123] 1.2 System configuration
[0124]
[0125] 1.3 Fluorescent PCR amplification
[0126] (1) Reaction system (30 μL): 20 μL of PCR amplification reaction solution + 10 μL of template D...
Embodiment 2
[0138] Embodiment 2 minimum detection limit test experiment
[0139] Using the pseudovirus extract with a concentration of 500copies / mL as a sensitivity template, according to the reaction procedure of Example 1, carry out amplification detection, repeat 20 reactions, and the reaction results are shown in Table 1:
[0140]
[0141] The results are shown in Table 1. When the pseudovirus concentration was 500 copies / mL, all 20 repeated experiments detected the new coronavirus and mutation sites, indicating that the minimum detection limit of this reagent is 500 copies / mL.
Embodiment 3
[0142] Example 3 Capability Verification Experiment for External Quality Assessment of Nucleic Acid Detection of the New Coronavirus Delta Mutant Strain
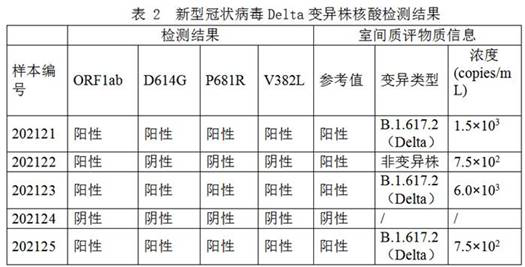
[0143] Use the above reagents to detect the extracted nucleic acid of the novel coronavirus Delta variant strain, and the results are shown in Table 2:
[0144]
[0145] The results are shown in Table 2. The detection results of the present invention for the nucleic acid detection of the new coronavirus delta variant fully meet the reference value requirements of the National Health and Medical Commission.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com