Preparation method of phenyl-containing compound
A compound and scheme technology, applied in the field of preparation of phenyl-containing compounds, can solve the problems of long reaction time, complex process, harsh conditions, etc., and achieve the effects of short reaction time, simple process and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
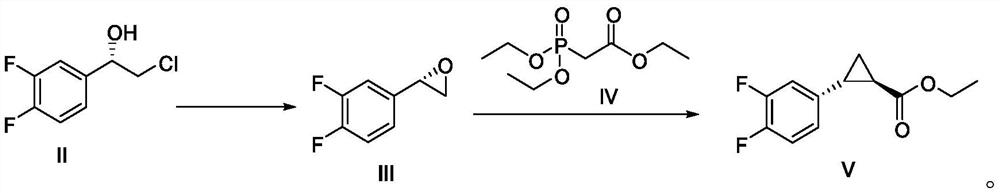
Embodiment 1
[0084] Preparation of Example 1 (2S)-2-(3,4-difluorophenyl)oxirane (Ⅲ)
[0085]
[0086] Add compound II (100 g, 0.52 mol) and toluene (500 mL) into a 1 L three-necked flask. With mechanical stirring, 312.5 g of 10% sodium hydroxide solution was added dropwise at 10-30°C, and the addition was completed in about 1 hour. T=20-25°C, heat preservation reaction for 5 hours, after HPLC monitoring, the raw material is less than 0.5%, then post-processing. The layers were separated, the aqueous layer was extracted once with toluene (200 mL), the organic layers were combined, and washed once with saturated aqueous sodium chloride solution. The organic layer was separated, and the compound III toluene solution was dried over anhydrous sodium sulfate, and directly put into the next step reaction without purification, and the HPLC purity was 97.2%.
Embodiment 2
[0087] Example 2 Preparation of (1R, 2R)-2-(3,4-difluorophenyl) ethyl cyclopropylcarboxylate (Ⅴ)
[0088]
[0089] Under nitrogen protection, the compound III toluene solution (about 700 mL) and sodium tert-butoxide (60.1 g, 0.62 mol, 1.2 eq) prepared above were added into a dry 1 L three-necked flask and stirred (suspension). Controlling the temperature at 20-30°C, compound IV (140.1g, 0.62mol, 1.2eq) was slowly added dropwise. After the dropwise addition, stir at T=20-30°C for 10 minutes, then raise the temperature to T=60°C for 48 hours. The reaction was quenched by monitoring compound III < 0.5% by HPLC. The reaction solution was cooled to T=5-10°C, and water (300 mL) was added dropwise. The layers were separated after standing, the upper organic layer was separated, the lower aqueous layer was extracted with toluene (150 mL) again, and the organic layers were combined. The organic layer was dried over anhydrous sodium sulfate and concentrated to dryness to obtain 12...
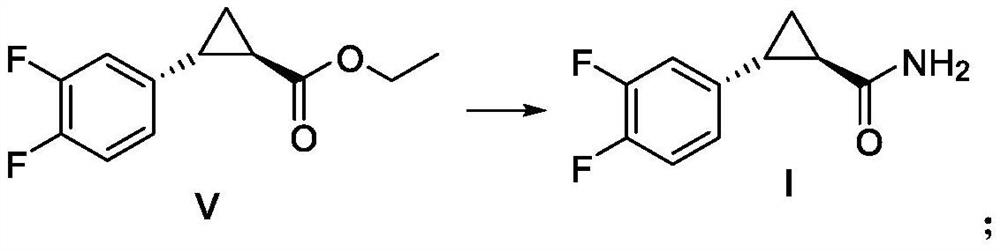
Embodiment 3
[0090] Example 3 Preparation of (1R, 2R)-2-(3,4-difluorophenyl) cyclopropylformamide (I)
[0091]
[0092] 200 mL of formamide, 42.2 g of sodium methoxide, and compound V (123.6 g of crude product) were successively added into a 1 L three-necked flask. Stir mechanically, heat up to T=60°C and react for 5h. After the reaction of the raw materials was complete, 600 mL of water was added dropwise under stirring, and an off-white solid was precipitated. After the dropwise addition was completed, the temperature was slowly lowered and cooled to T=0-5°C for crystallization for 3 hours. Filter and wash the filter cake with purified water (200 mL). The solid was dried in a forced air drying oven at 50°C to constant weight to obtain an off-white solid Compound I (88.4 g, chemical purity 98.5%, yield 86.2%, yield calculated as Compound II). 1 H NMR (DMSO-d 6 )δ: 1.16~1.23(m,1H), 1.29~1.34(m,1H), 1.79~1.85(m,1H), 2.24~2.26(m,1H), 5.73(s,2H), 6.79~6.87( m,2H),6.89~7.01(m,1H); 13 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com