Artificial tears
A technology of liquid and liquid lipids, applied in the direction of drug combination, non-active ingredients of oil/fat/wax, microcapsules, etc., which can solve the problems of additive toxicity, vision, blurring, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 - Size Measurement and Zeta Potential Measurement:
[0049] Chain diameter and Zeta potential NLC via DLS. Characterization indicates that the cholesterol-castor oil nanostructure lipid carrier (CHCACO-NLC) ratio is 1: 1 sample is most stable. The size and zeta potential of CHCAO-NLC are shown in Table 1. Sample particle size (nm) Zeta potential (MV)
[0050] Table 1. The size and zeta potential of thechcao-NLC.
[0051] sample name Particle size (nm) Zeta Potential (MV) CHCAO1: 1 304 -8.5
Embodiment 2
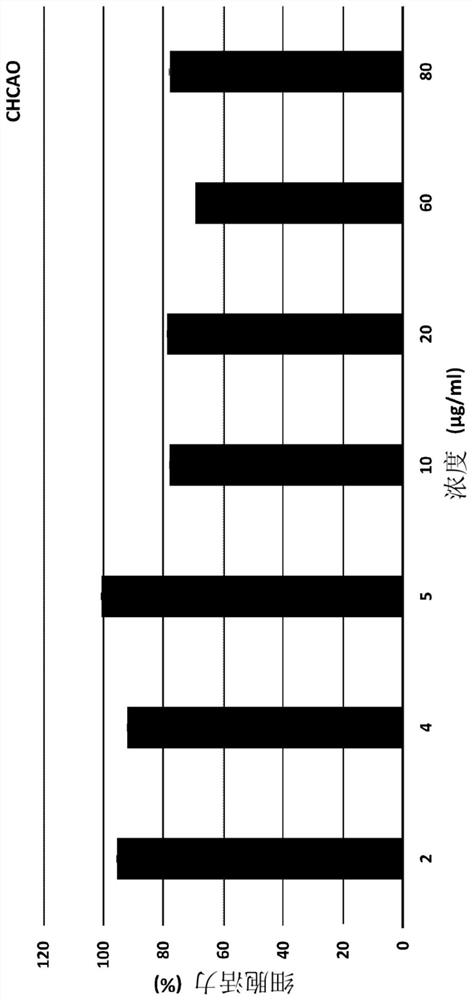
[0052] Example 2 - Cytotoxicity for prepared chcaco-NLC:
[0053] CHCAO-NLC cytotoxicity was carried out on Human Corneal Epithelium Cells, Hcec. Cells of 10,000 cells / well were inoculated in a 96-well plate, and then cultured for 24 hours in DMEM medium containing 10% FBS. The cells were treated with different concentrations of CHCAO-NLC and cultured with CHCAO-NLC treated with CHCAO-NLC in a moisture of 5% CO2 at 37 ° C for 4 hours. After 4 hours, the medium was replaced with fresh medium, and the cells were maintained for more than 20 hours. After the specified time, MTT (3- (4,5-dimethylthiazole-2-yl) -2,5-diphenyl bromide) reagent was added to each well, and the cells were cultured for 4 hours. The medium in each well was replaced with 200 μl of DMSO to dissolve the crystallization. Record the absorbance (O.D.) with the enzyme label instrument. Calculate the cell viability by using the following equation:
[0054] Cell viability (%) = [o.d. (test) / o.d. (Control)] x100
[0...
Embodiment 3
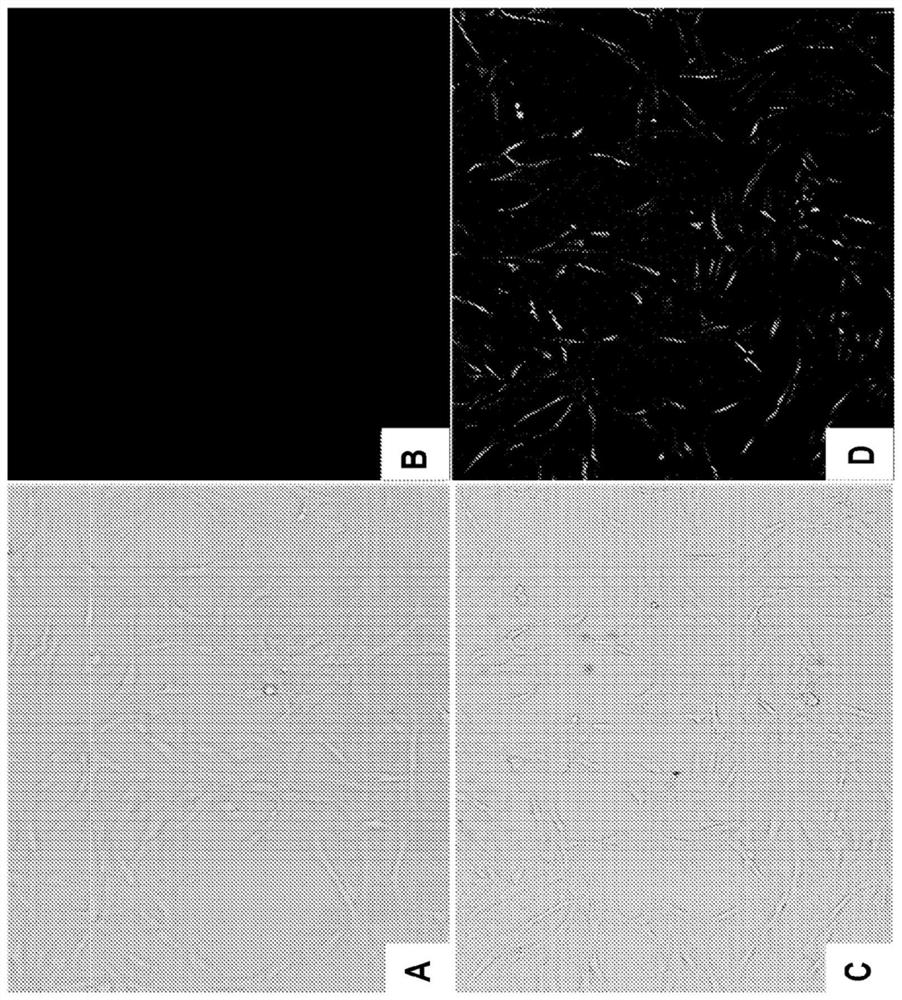
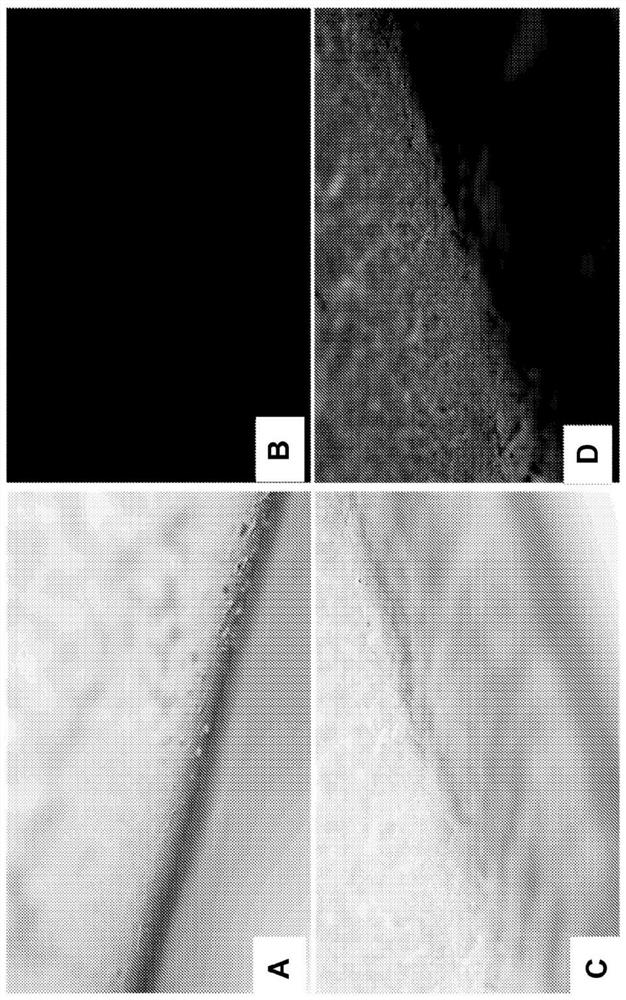
[0057] Example 3 - Cell Patient Research
[0058] The use of fluorescence microscopy further examines CHCAO-NLC in vitro catalytes in vitro. 5 × 10 per dish 4 The density of a cell is inoculated in a cop-focused impeva. The chcaO-NLC-6 labeled CHCAO-NLC was prepared and exposed to the HCT cells in the medium. After 4 hours at 37 ° C, the medium treatment treated with CHCAO-NLC was removed and the cells were washed. Further cellular intake of cells under fluorescence microscope. The data shows that NLC is internalized in cells, and the coumarin-6 encapsulated in CHCAO-NLC is successfully released into the cytoplasm of HCT cells. Detecting dexamethasone in CHCAO-NLC will be released within HCEC cells ( figure 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com