Medicinal composition containing roxadustat solid dispersion and preparation method of medicinal composition
A technology of solid dispersion and roxadustat, which can be applied to medical preparations containing active ingredients, medical preparations without active ingredients, and drug combinations, etc. The problems of poor stability and undisclosed preparation process can achieve the effects of good photostability, increased solubility, and improved in vitro dissolution rate and bioavailability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
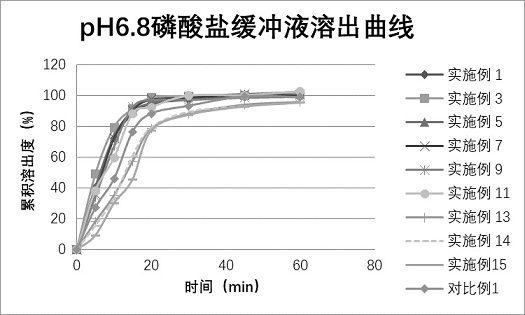
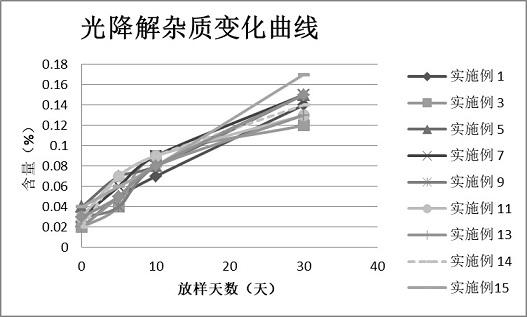
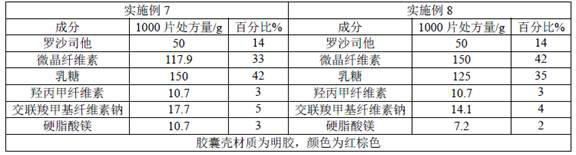
Image
Examples
Embodiment 1-2
[0033] Table 1 Example 1-2 prescription list
[0034]
[0035] Embodiment 1-2 preparation method
[0036] ① According to the above prescription, ultrasonically dissolve the prescribed amount of roxadustat and povidone in 100g of 50% ethanol solution at the same time as the drug-containing carrier solution. The drug-containing carrier solution is a light yellow transparent liquid without lumps and bubble;
[0037] ② Take the prescription amount of croscarmellose sodium and microcrystalline cellulose and mix them in a fluidized bed for 5 minutes;
[0038] ③Continue to spray the drug-containing carrier solution in step 1, and obtain a solid dispersion containing roxadustat through one-step granulation, and dry (moisture content 1-3%);
[0039] ④Sizing and blending: 30-mesh sizing, then put the dry granules in a three-dimensional mixer, add magnesium stearate for total blending, set the parameters at 12rpm, and mix for 5 minutes;
[0040] ⑤Filling: Fill the total mixed granu...
Embodiment 3-4
[0042] Table 2 Example 3-4 prescription list
[0043]
[0044] Embodiment 3-4 preparation method
[0045] ① According to the above prescription, ultrasonically dissolve the prescribed amount of roxadustat and povidone in 100g of 50% ethanol solution at the same time as the drug-containing carrier solution. The drug-containing carrier solution is a light yellow transparent liquid without lumps and bubble;
[0046] ② Take the prescribed amount of croscarmellose sodium, microcrystalline cellulose and lactose and mix them in a fluidized bed for 5 minutes;
[0047] ③Continue to spray the drug-containing carrier solution in step 1, and obtain a solid dispersion containing roxadustat through one-step granulation, and dry (moisture content 1-3%);
[0048] ④Sizing and blending: 30-mesh sizing, then put the dry granules in a three-dimensional mixer, add magnesium stearate for total blending, set the parameters at 12rpm, and mix for 5 minutes;
[0049] ⑤Filling: Fill the total mixe...
Embodiment 5-6
[0051] Table 3 Example 5-6 prescription list
[0052]
[0053] Embodiment 5-6 preparation method
[0054] ① According to the above prescription, ultrasonically dissolve the prescribed amount of roxadustat and hypromellose in 100 g of 50% ethanol solution at the same time as the drug-containing carrier solution. The drug-containing carrier solution is a light yellow transparent liquid without lumps blocks and bubbles;
[0055] ②Take the prescribed amount of croscarmellose sodium and lactose in a fluidized bed and mix for 5 minutes;
[0056] ③Continue to spray the drug-containing carrier solution in step 1, and obtain a solid dispersion containing roxadustat through one-step granulation, and dry (moisture content 1-3%);
[0057] ④Sizing and blending: 30-mesh sizing, then put the dry granules in a three-dimensional mixer, add magnesium stearate for total blending, set the parameters at 12rpm, and mix for 5 minutes;
[0058] ⑤Filling: Fill the total mixed granules to obtain ro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com