Antibacterial medical drainage polyurethane foam and preparation method thereof
A polyurethane foam and foam stabilizer technology, applied in the field of medical materials, can solve the problems of increasing the burden on patients, multi-drug-resistant bacterial infection of patients, infection risks, etc., achieve good antibacterial and antibacterial performance, and solve the effect of anaerobic bacteria breeding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
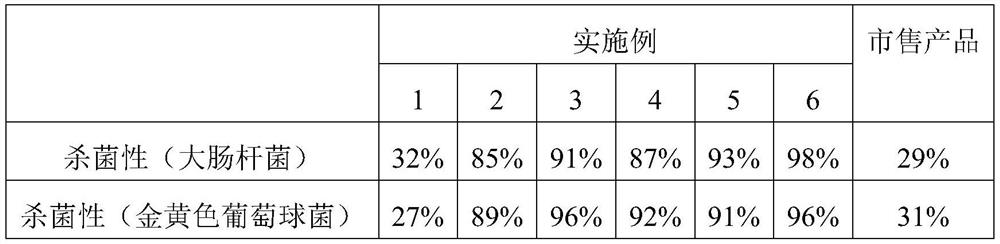
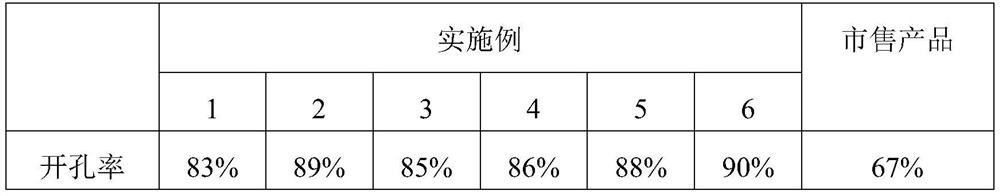
Embodiment 1
[0023] The preparation method of antibacterial type medical drainage polyurethane foam 1 comprises the following steps:
[0024] 1) Weigh 50 parts of polyether polyols with a hydroxyl value of 55 mg KOH / g and a molecular weight of 4300 according to the ratio of parts by weight, 50 parts of polyether polyols with a hydroxyl value of 103 mg KOH / g and a molecular weight of 3700, and evenly 2.5 parts of foaming agent, 10 parts of dichloromethane, 55 parts of toluene diisocyanate, 0.5 parts of triethylenediamine, 0.5 parts of zinc catalyst, 5 parts of water, for later use;
[0025] 2) Mechanically mix polyether polyol, silicone foam stabilizer, methylene chloride, tannic acid, imidazolidinyl urea, silver sulfadiazine, chitosan and toluene diisocyanate to obtain a white material, which is the pre-mixture. Polymer;
[0026] 3) Pour triethylenediamine, zinc catalyst and water into the prepolymer, mechanically stir and mix evenly, pour into a mold to foam, mature at 80° C. for 24 hour...
Embodiment 2
[0028] The preparation method of antibacterial type medical drainage polyurethane foam 2 comprises the following steps:
[0029] 1) Weigh 50 parts of polyether polyols with a hydroxyl value of 55 mg KOH / g and a molecular weight of 4300 according to the ratio of parts by weight, 50 parts of polyether polyols with a hydroxyl value of 103 mg KOH / g and a molecular weight of 3700, and evenly 2 parts of foaming agent, 8 parts of cyclopentane, 0.5 part of tannic acid, 0.5 part of imidazolidinyl urea, 1 part of silver sulfadiazine, 6 parts of chitosan, 55 parts of toluene diisocyanate, 0.2 part of ethylenediamine, zinc 0.2 parts of catalyst, 4.5 parts of water, set aside;
[0030] 2) Mechanically mix polyether polyol, silicone foam stabilizer, cyclopentane, tannic acid, imidazolidinyl urea, silver sulfadiazine, chitosan and toluene diisocyanate to obtain a white material, which is the prepared Polymer;
[0031] 3) Pour ethylenediamine, zinc catalyst and water into the prepolymer, me...
Embodiment 3
[0033] The preparation method of antibacterial type medical drainage polyurethane foam 3 comprises the following steps:
[0034] 1) Weigh 30 parts of polyether polyols with a hydroxyl value of 55 mg KOH / g and a molecular weight of 4300 according to the ratio of parts by weight, 70 parts of polyether polyols with a hydroxyl value of 103 mg KOH / g and a molecular weight of 3700, and evenly 1.5 parts of foaming agent, 10 parts of cyclopentane, 0.1 part of tannic acid, 0.5 part of imidazolidinyl urea, 2 parts of silver sulfadiazine, 5 parts of chitosan, 45 parts of toluene diisocyanate, 0.3 part of triethylamine, zinc 0.2 parts of catalyst, 5 parts of water, spare;
[0035] 2) Mechanically mix polyether polyol, silicone foam stabilizer, cyclopentane, tannic acid, imidazolidinyl urea, silver sulfadiazine, chitosan and toluene diisocyanate to obtain a white material, which is the prepared Polymer;
[0036] 3) Pour triethylamine, zinc catalyst and water into the prepolymer, mechanic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hydroxyl value | aaaaa | aaaaa |
| hydroxyl value | aaaaa | aaaaa |
| hydroxyl value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com