Copper-based catalyst for hydrogen production by methanol steam reforming and preparation method and application thereof
A technology of steam reforming and copper-based catalysts, which is applied in the direction of catalyst activation/preparation, chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, etc., which can solve the problems of high hydrogen selectivity, poor high temperature stability, Time-consuming preparation process and other issues, to achieve the effect of good low-temperature activity, strong thermal stability, simple and easy-to-operate preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
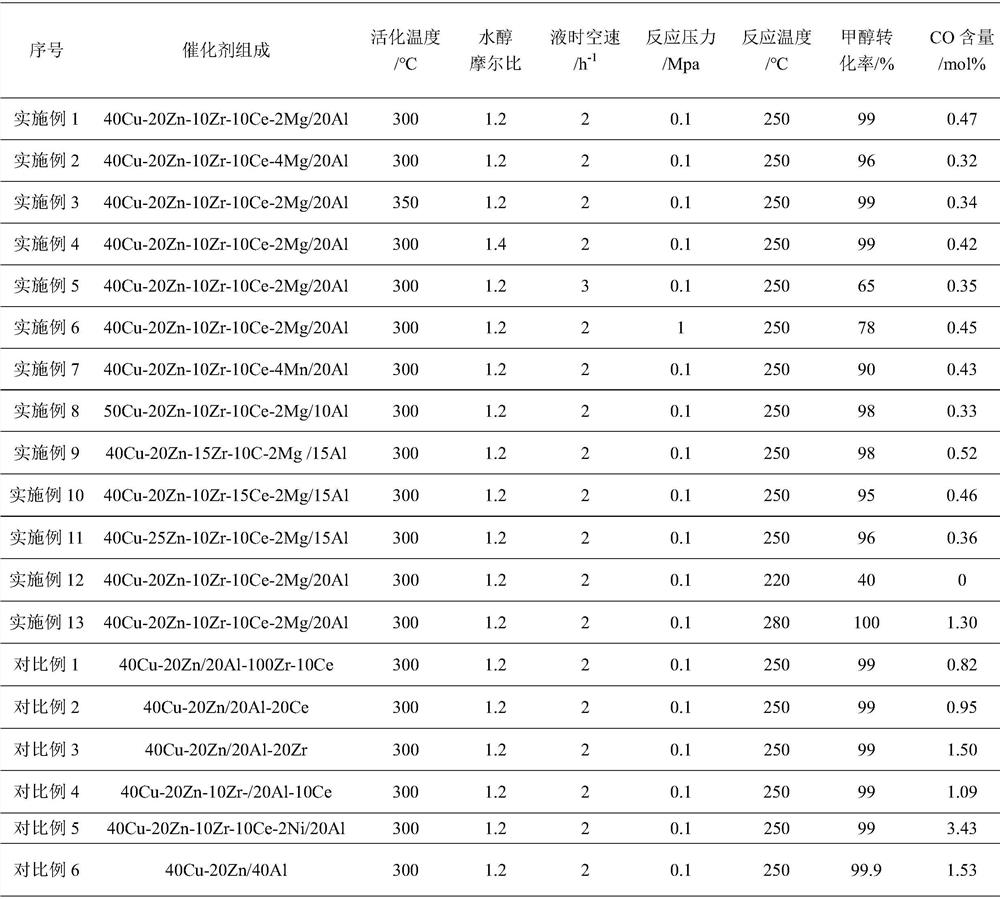
[0031] (1) Dissolve 14.742g copper nitrate, 9.138g zinc nitrate, 6.639g cerium nitrate, 6.604g zirconium nitrate, 0.787g magnesium nitrate in 100mL deionized water and stir to clear liquid to obtain solution A; dissolve 11.537g aluminum nitrate Dissolve 21.2 g of sodium carbonate in 100 mL of deionized water to obtain a sodium carbonate solution; dissolve 11.3 g of ammonium bicarbonate in 100 mL of deionized water to obtain an ammonium bicarbonate solution;
[0032] (2) at 70-80 ℃, add solution A to sodium carbonate solution to carry out reverse co-precipitation, keep fully stirring during precipitation, obtain mixed mother liquor, and adjust the pH of mixed mother liquor to 7.5-8.0; at 45-55 ℃ , adding the ammonium bicarbonate solution to solution B for precipitation, maintaining full stirring during the precipitation to obtain an aluminum emulsion, and adjusting the pH of the aluminum emulsion to 6.5-7.0;
[0033] (3) At 70-80 ℃, the aluminum emulsion is added to the mixed m...
Embodiment 2
[0036] (1) Dissolve 14.742g copper nitrate, 9.138g zinc nitrate, 6.639g cerium nitrate, 6.604g zirconium nitrate, 1.575g magnesium nitrate in 100mL deionized water and stir to clear liquid to obtain solution A; dissolve 11.537g aluminum nitrate Dissolve 21.2 g of sodium carbonate in 100 mL of deionized water to obtain a sodium carbonate solution; dissolve 11.3 g of ammonium bicarbonate in 100 mL of deionized water to obtain an ammonium bicarbonate solution;
[0037] Steps (2) to (3) are the same as in Example 1; the catalyst is labeled as 40Cu-20Zn-10Zr-10Ce-4Mg / 20Al.
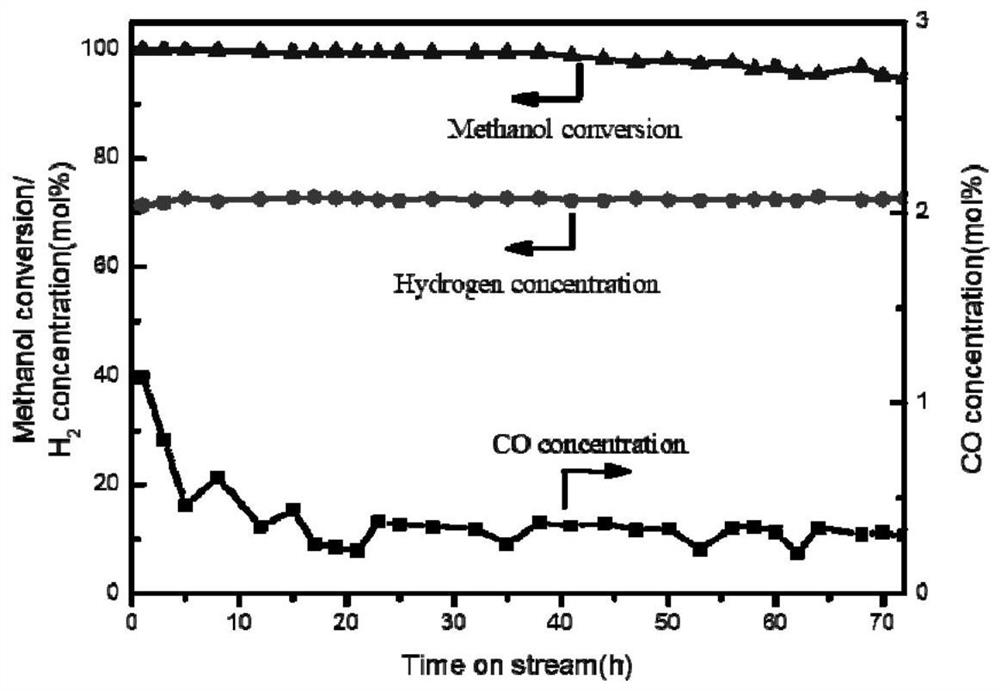
[0038] The copper-based catalyst for hydrogen production by methanol steam reforming prepared in this example is used for the hydrogen production reaction of fixed-bed methanol steam reforming. The reaction activation temperature is 300 ° C, the raw material water-alcohol molar ratio is 1.2, and the liquid hourly space velocity is 2 h. -1 , the reaction pressure is 0.1Mpa, the reaction temperature is 250 ℃, and...
Embodiment 3
[0040] Steps (1) to (3) are the same as in Example 1; the catalyst is labeled as 40Cu-20Zn-10Zr-10Ce-2Mg / 20Al.
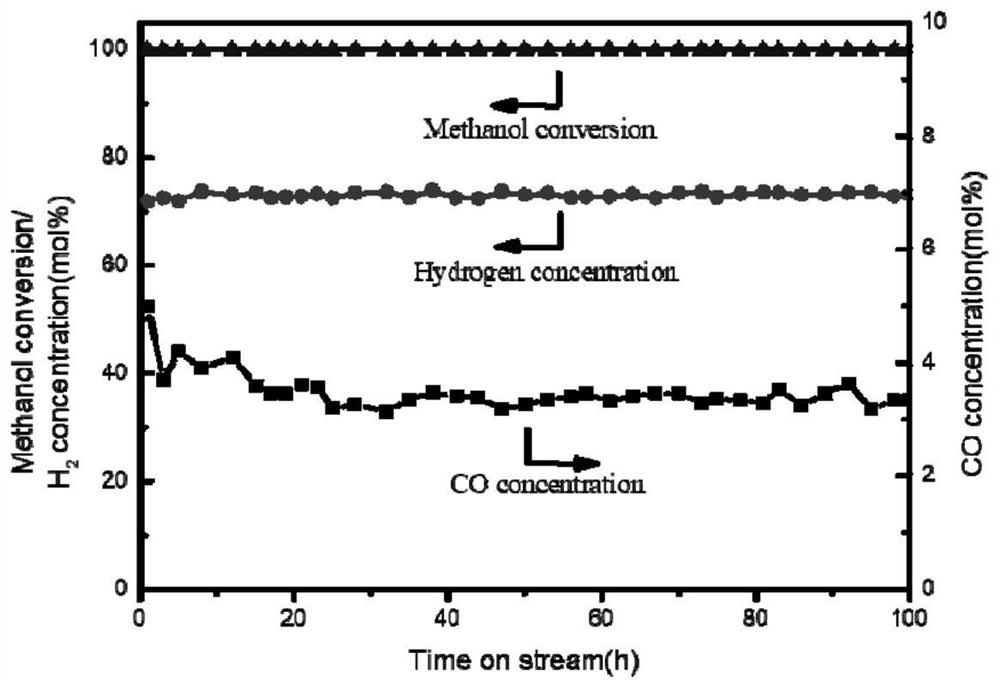
[0041] The copper-based catalyst for hydrogen production by methanol steam reforming prepared in this example is used for the hydrogen production reaction of fixed-bed methanol steam reforming. The reaction activation temperature is 350 ° C, the raw material water-alcohol molar ratio is 1.2, and the liquid hourly space velocity is 2 h. -1 , the reaction pressure is 0.1Mpa, the reaction temperature is 250℃, and the reaction time is 8h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com