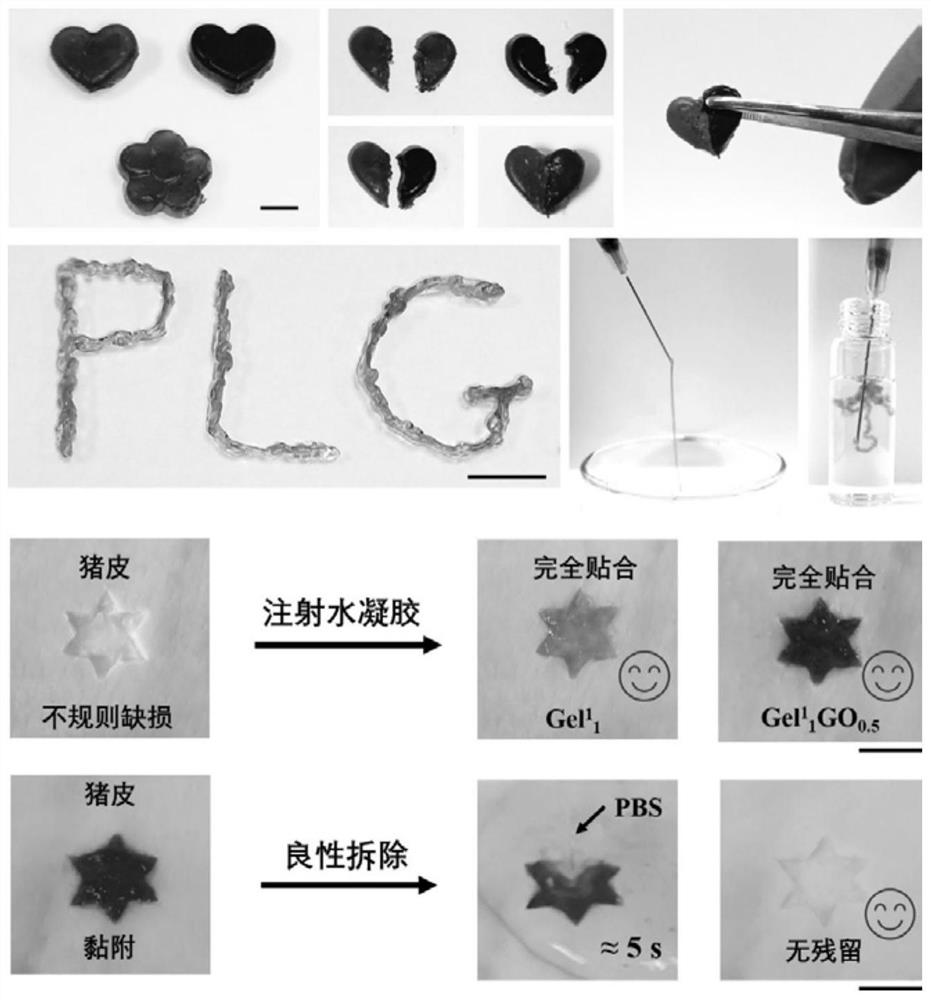
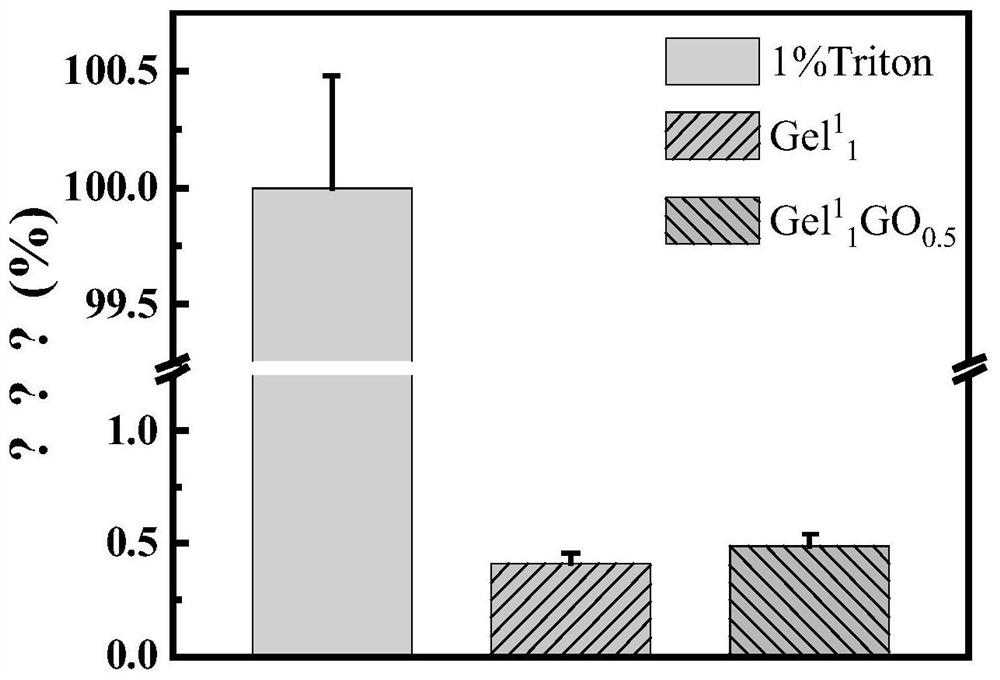
Ultralow-concentration single-component polypeptide hydrogel as well as preparation method and application thereof
An ultra-low concentration, hydrogel technology, applied in the fields of application, medical science, surgery, etc., can solve the problems of lack of tissue interface clearance, difficult application, long hemostasis time, etc., and achieve high practical application value and excellent biocompatibility Sexuality, the effect of suitable adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0055] The present invention also provides a method for preparing an ultra-low concentration single-component polypeptide hydrogel, comprising the following steps:
[0056] (1) Using carboxylated polypeptide as the polymer skeleton, the catechol-functionalized polypeptide is prepared after catechol modification;
[0057] (2) oxidizing the catechol-functionalized polypeptide obtained in step (1) in an alkaline solution, and then dialyzing or stirring with deionized water;
[0058] (3) Freeze the solution obtained in step (2), thaw at room temperature to obtain an ultra-low concentration single-component polypeptide hydrogel or freeze-dry the solution obtained in step (2) to obtain a solid, and then The polypeptide hydrogel is obtained after the solution is dissolved, and the gelation time is 20-120s.
[0059] In one embodiment of the present invention, the catechol is dopamine or 3,4-dihydroxybenzylamine, etc., and the grafting rate is 10%-50%.
[0060] In one embodiment of t...
Embodiment 1
[0073] This example provides a method for preparing an ultra-low concentration single-component polypeptide hydrogel.
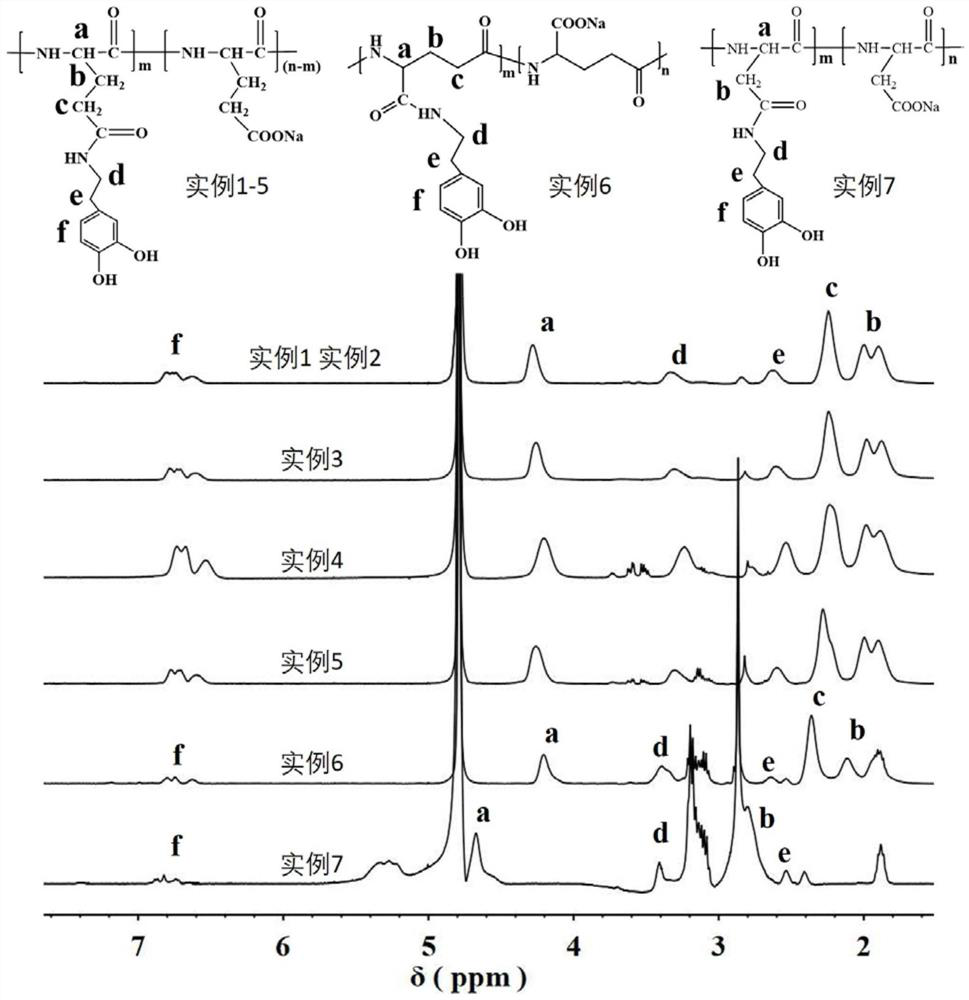
[0074] (1) Dissolve 129 mg (1 mmol) of α-polyglutamic acid (molecular weight 38.7 kDa) with a degree of polymerization of 300 in DMSO (dimethyl sulfoxide), and add 46 mg of 1-(3-dimethylaminopropyl) at low temperature base)-3-ethylcarbodiimide hydrochloride (EDC) (0.24mmol) and 27.6mg N-hydroxysuccinimide (NHS) (0.24mmol) activated overnight, under nitrogen atmosphere, add dopamine hydrochloride 38mg (0.2mmol) and a small amount of triethylamine (TEA) as an acid-binding agent, and reacted at 25°C for 48h, and then the reaction solution was directly dialyzed in PBS with pH=7.4 for 1d, then dialyzed with deionized water for 1d, and then deionized After water dialysis for 1 day, after using ultraviolet light to detect the absence of dopamine (DA) in water, the obtained colorless transparent solution was collected and freeze-dried to obtain a white solid, that is...
Embodiment 2
[0079] This example provides a method for preparing a hybrid polypeptide hydrogel.
[0080] (1) Dissolve 129 mg (1 mmol) of α-polyglutamic acid with a degree of polymerization of 300 in DMSO, add 46 mg EDC (0.24 mmol) and 27.6 mg NHS (0.24 mmol) to activate overnight at low temperature, and add dopamine salt under nitrogen atmosphere Salt 38mg (0.2mmol) and a small amount of TEA were used as acid-binding agent, and after reacting at 25°C for 48h, the reaction solution was directly dialyzed with deionized water, and the resulting solution was collected after using ultraviolet light to detect the absence of DA in the water.
[0081] The schematic diagram of catechol functionalized α-polyglutamic acid H NMR spectrum obtained in this example is as follows figure 1 shown.
[0082] (2) The solution obtained in (1) was dissolved in 0.05M NaHCO 3 Dialyze in the solution for 24 hours at a temperature of 25°C, then dialyze in deionized water for 6 hours to remove NaHCO 3 .
[0083] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com