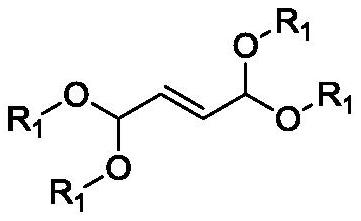
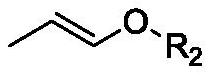
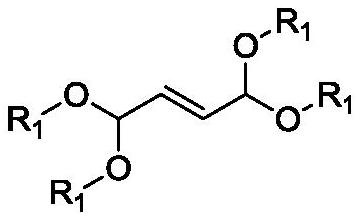
Method for preparing 1, 8-dialkoxy-1, 3, 6, 8-tetraalkoxy-2, 7-dimethyl-4-octylene
A technology of tetraalkoxy and dialkoxy, which is applied in the field of organic synthesis, can solve the problems of large environmental pollution, high production cost, corrosion, etc., and achieve the effect of simple post-processing and improved reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Preparation of a 1,1,3,6,8,8-hexamethoxy-2,7-dimethyl-4-octene
[0038] Add 0.1mol (17.9g) 1,1,4,4-tetramethoxy-2-butene, 0.21mol (15.2g) propenyl methyl ether, 0.01mol (1.66g) potassium iodide, 0.33 g tetraethylammonium hexafluorophosphate and 178.8 g solvent (143.0 g ethyl acetate and 35.8 g water). Two graphite rods (Φ5mm) were used as anode and cathode respectively. Pass the reaction mixture through 50mA / cm 2 The electric current, stirred at 20 ℃ for 6.0h. After the reaction is completed, add it to a separatory funnel and let it stand for stratification, and the organic phase rotary evaporator will remove the solvent to obtain 1,1,3,6,8,8-hexamethoxy-2,7-dimethyl -4-octene product, the reaction conversion rate is 95.0%, the selectivity is 98.3%, and the reaction yield is 93.4%.
[0039] The H NMR spectrum of 1,1,3,6,8,8-hexamethoxy-2,7-dimethyl-4-octene:
[0040] HNMR (DMSO, 400 MHz) δ = 1.53 (s, 6H), 2.58 (s, 2H), 3.31 (s, 18H), 5.42 (s, 2H), 6.88 (...
Embodiment 2
[0041]Example 2: Preparation of a 1,8-diethoxy-1,3,6,8-tetramethoxy-2,7-dimethyl-4-octene
[0042] Add 0.1mol (17.9g) 1,1,4,4-tetramethoxy-2-butene, 0.23mol (19.8g) propenyl ethyl ether, 0.005mol (0.75g) sodium iodide, 0.37 g tetraethylammonium hexafluorophosphate and 178.8 g solvent (158.9 g methanol and 19.9 g water). Two graphite rods (5 mm in diameter) were used as anode and cathode, respectively. Pass the reaction mixture through 100mA / cm 2 The electric current, stirred at 30 ℃ for 4.0h. After the reaction is completed, add it to a separatory funnel and let it stand for stratification, and remove the solvent with a rotary evaporator for the organic phase to obtain 1,8-diethoxy-1,3,6,8-tetramethoxy-2 , 7-dimethyl-4-octene product, the reaction conversion rate is 94.6%, the selectivity is 96.3%, and the reaction yield is 91.1%.
Embodiment 3
[0043] Example 3: Preparation of a 1,1,3,6,8,8-hexamethoxy-2,7-dimethyl-4-octene
[0044] Add 0.2mol (35.7g) 1,1,4,4-tetramethoxy-2-butene, 0.5mol (36.0g) propenyl methyl ether, 0.04mol (6.64g) potassium iodide, 0.66 g tetraethylammonium hexafluorophosphate and 214.2 g solvent (194.7 g methanol and 19.5 g water). Two graphite rods (Φ5mm) were used as anode and cathode respectively. Pass the reaction mixture through 60mA / cm 2 The electric current, stirred at 50 ℃ for 2.0h. After the reaction is completed, add it to a separatory funnel and let it stand for stratification, and the organic phase rotary evaporator will remove the solvent to obtain 1,1,3,6,8,8-hexamethoxy-2,7-dimethyl -4-octene product, the reaction conversion rate is 93.5%, the selectivity is 96.4%, and the reaction yield is 90.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com