Preparation method of optically pure lobeline hydrochloride and enantiomer thereof
A technology of lobeline hydrochloride and enantiomers, which is applied in the field of preparation of optically pure lobeline hydrochloride and its enantiomers, can solve the problems of complex preparation of chiral catalysts, low yield of enzymatic reactions, and ligand Expensive and other issues, to achieve the effect of being suitable for large-scale industrial production, saving process costs, and high optical purity of products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1 A kind of preparation method of optically pure lobeline hydrochloride and its enantiomer
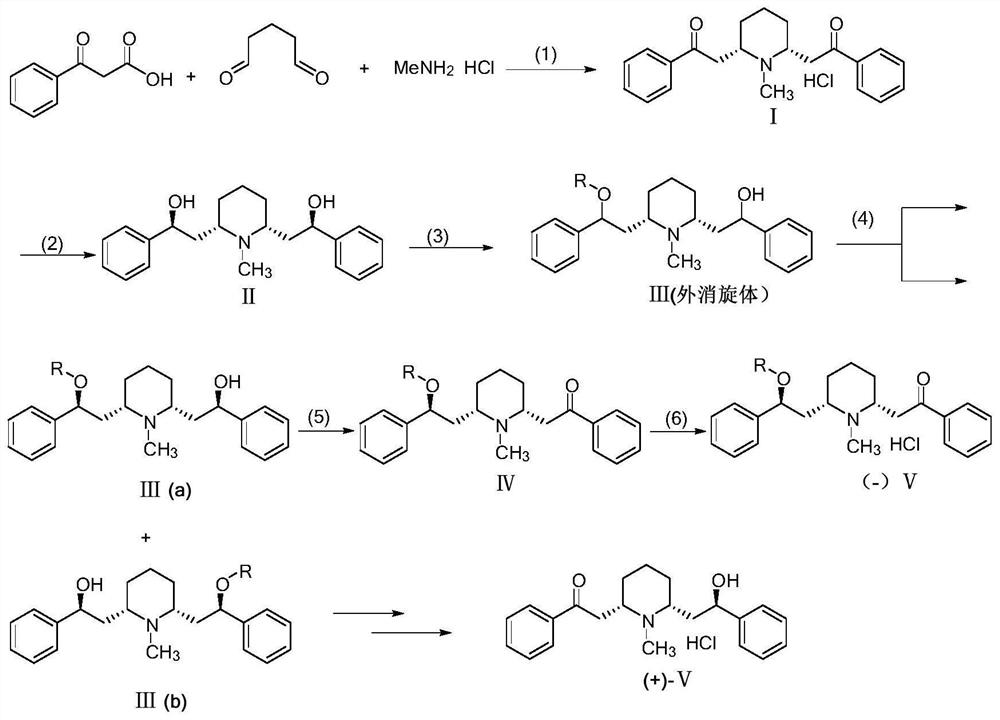
[0042] The preparation method of the optically pure lobeline hydrochloride and its enantiomers, the reaction scheme is as follows:
[0043]
[0044] Specifically include the following steps:
[0045] (1) Condensation reaction: Dissolve 13.12g (0.08mol) of benzoylacetic acid, 2.72g (0.04mol) of methylamine hydrochloride, and 8.0mL (0.04mol) of 50% glutaraldehyde aqueous solution in 600mL of citric acid buffer solution (0.05M, pH=4.0), stirred and reacted at room temperature for 48 hours, washed with n-hexane (500mL×2) after the reaction, then cooled the water phase to below 0°C, acidified to pH with 4mol / L HCl aqueous solution =1~2, and stirred to form a white precipitate, the white precipitate was filtered out with a Buchner funnel and the filter cake was washed with a small amount of water, dried in vacuo to obtain behenone hydrochloride Ⅰ (8.2g, yield: 55.1%) ;...
Embodiment 2
[0060] Embodiment 2 A kind of preparation method of optically pure lobeline hydrochloride and its enantiomer
[0061] Compared with the preparation method of Example 1, the difference of the preparation method of Example 2 is that the step (4) splits and specifically adopts the following steps:
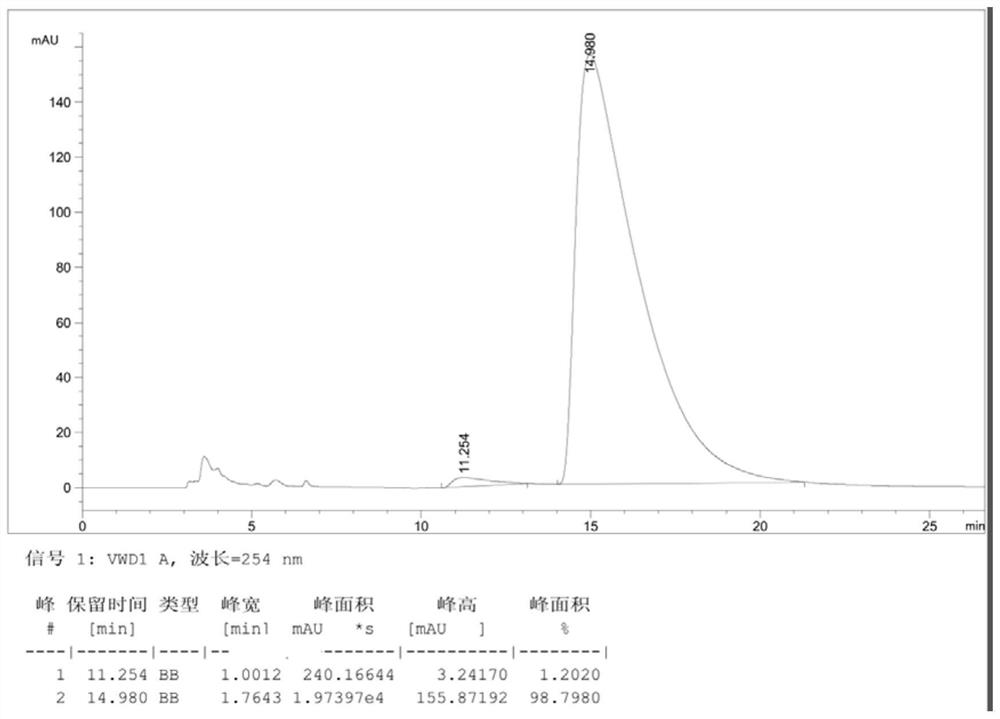
[0062] Dissolve compound III (racemate) (3.95g, 0.01mol) obtained in step (3) in 40mL ethanol at room temperature, stir and heat to 80°C, after dissolving, add resolving agent (-)-camphor- 10-sulfonic acid (1.28g, 0.0055mol), continue to stir until dissolving and cool down, until white solid is separated out, after stirring for 30 minutes, suction filtration obtains the salt 2.10g that compound III (a) forms with resolving agent, yield is 33.5%, ee value is close to 100% (the HPLC analysis spectrogram of obtained product III (a) sees Figure 4 , wherein, the peak time of Ⅲ(a) is 14.2min).
[0063] Refer to Example 1 for other operations and parameters.
Embodiment 3
[0064] Embodiment 3 A kind of preparation method of optically pure lobeline hydrochloride and its enantiomer
[0065] Compared with the preparation method of Example 1, the difference of the preparation method of Example 3 is that the step (4) splitting specifically adopts the following steps:
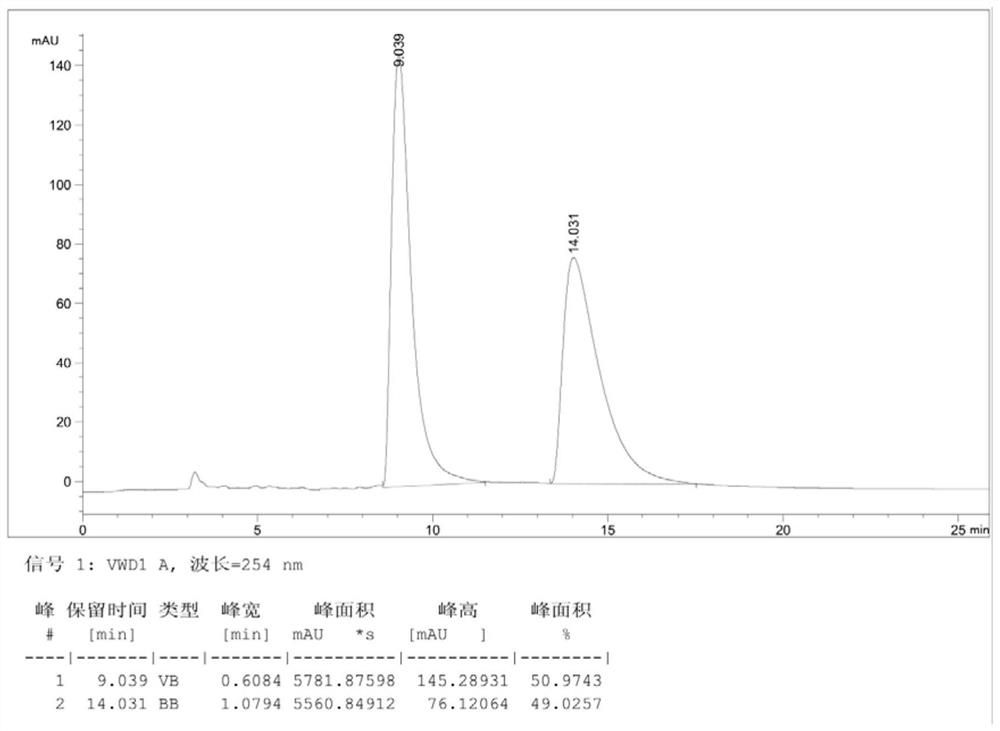
[0066] Dissolve compound III (racemate) (3.95g, 0.01mol) obtained in step (3) in 30mL toluene at room temperature, stir and heat to 80°C, after dissolving, add the resolving agent di-p-methoxybenzene Formyl-L-tartaric acid (2.30g, 0.0055mol), continued to stir until dissolved and then cooled until a white solid was precipitated. After stirring for 60 minutes, suction filtered to obtain 2.59g of the salt formed by compound III (a) and a resolving agent. The rate is 31.9%, and the ee value is 87% (the HPLC analysis spectrogram of gained product III (a) sees Figure 5 , wherein, the peak time of III(a) is 13.9min, and the peak time of III(b) is 9.5min).
[0067] Refer to Example 1 for o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com