L-asparaginase sala and its encoding gene and application
An asparaginase and gene technology, applied in the application, genetic engineering, plant genetic improvement and other directions, can solve the problems of limiting the wide application of L-asparaginase, narrow temperature/pH range of activity, poor stability, etc. The effect of wide reaction temperature, wide reaction pH and high enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
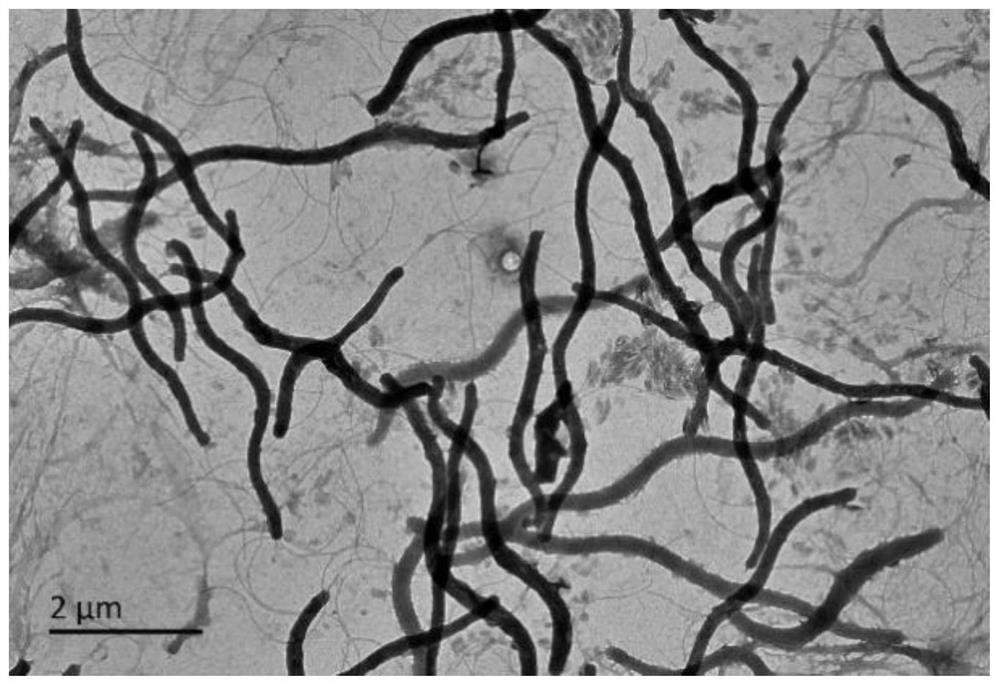
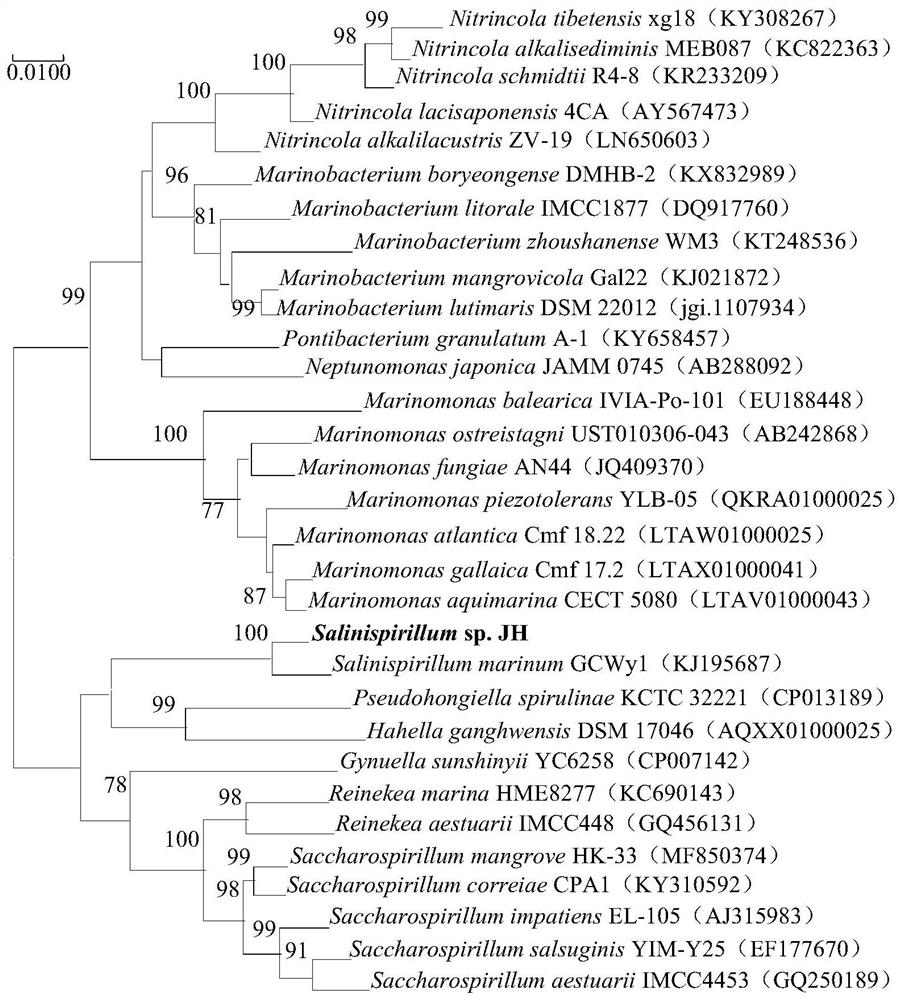
[0020] Example 1. Separation, identification and preservation of Halospira JH
[0021] 1. Separation
[0022] The sample was taken from an alkaline lake in the Ordos area of Inner Mongolia, 50 μL of the alkaline lake sample was taken, 450 μL of the alkaline lake filtrate was added, and 10 was obtained by pipetting and mixing. -1 concentration samples, diluted sequentially to obtain 10 -1 , 10 -2 , 10 -3 , 10 -4 , 10 -5 concentration of the sample. Take 20 μL, 40 μL of alkaline lake water sample stock solution and the samples obtained by dilution, spread them into LBH solid medium, and cultivate them in a constant temperature incubator at 35 °C for 3-4 d to observe the growth.
[0023] 2. Identification
[0024] The purified strains were inoculated into LBH solid medium and incubated at a constant temperature of 30°C for 3 days, and the colony color, bulge, edge regularity, size and transparency of the strains were recorded. The morphology, size and extracellular appe...
Embodiment 2
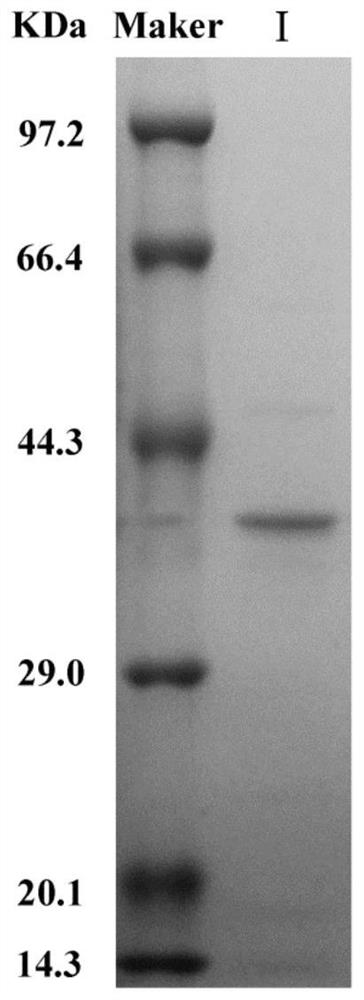
[0034] Example 2. Preparation of L-asparaginase (SaLA protein)
[0035] After extensive sequence analysis, alignment and functional verification, a new protein was discovered from Halospira JH, which was named SaLA protein, as shown in sequence 1 of the sequence listing. The gene encoding SaDL protein in Halospira JH was named SaLA gene, and its coding frame is shown in sequence 2 of the sequence listing.
[0036] 1. Construction of recombinant plasmids
[0037] 1. Using the genomic DNA of Halospira JH as a template, PCR amplification was carried out using a primer pair composed of LA-F and LA-R, and the PCR amplification product was recovered.
[0038] LA-F: 5'- CCGG AATTCATGGACACC-3';
[0039] LA-R: 5'-GCCAAGCTTTTACTGGTGCA-3'.
[0040] 2. Take the PCR amplification product obtained in step 1 and connect it with the pET-28a vector to obtain the recombinant plasmid pET-28a-SaLA.
[0041] pET-28a Vector (pET-28a Vector): Anorum (Beijing) Biotechnology Co., Ltd., catalog num...
Embodiment 3
[0054] Example 3. Enzymatic properties of L-asparaginase (SaLA protein)
[0055] PBS buffer (50mM, pH 8.0): Weigh 1.44g sodium dihydrogen phosphate, 0.24g potassium dihydrogen phosphate, 0.20g potassium chloride, 8.00g sodium chloride, dissolve in 800mL ultrapure water, adjust pH with HCl to 8.0, and make up to 1L.
[0056] Substrate solution (25mM asparagine): Weigh 3.303g of L-asparagine, dissolve in PBS buffer, and make up to 1L.
[0057] 1. The effect of pH on the activity of L-asparaginase
[0058] 1. Optimum pH
[0059] The SaLA protein solution prepared in Example 2 was taken and diluted with PBS buffer (50 mM, pH 8.0) to 2 times the volume, and the diluted solution was used as the test solution.
[0060] Detection method: Add 200 μL of test solution and 700 μL of substrate solution (prepared with pH buffer), react at 37°C for 15 minutes, add 100 μL of 15% TCA (trichloroacetic acid) to terminate the reaction, centrifuge at 10,000 × g for 10 minutes, and then take the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com