Nitroimidazole derivative and preparation method and application thereof
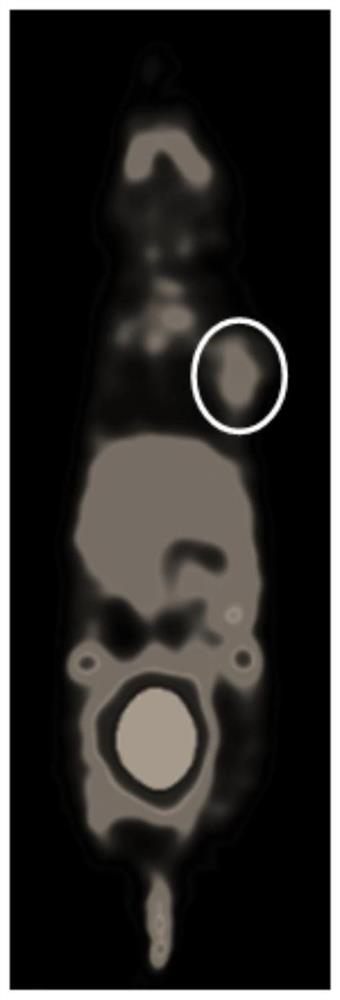
A technology for nitroimidazoles and nitroimidazoles, which is applied in the field of nitroimidazole derivatives and their preparation, can solve problems such as poor pharmacokinetics, and achieves improved pharmacokinetic properties, improved signal-to-noise ratio, High intake effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
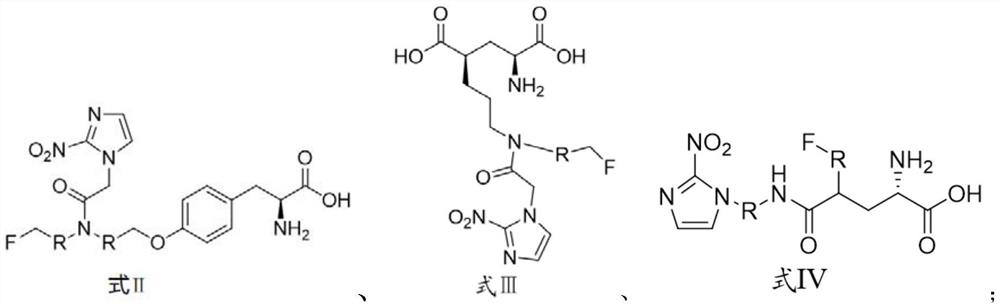
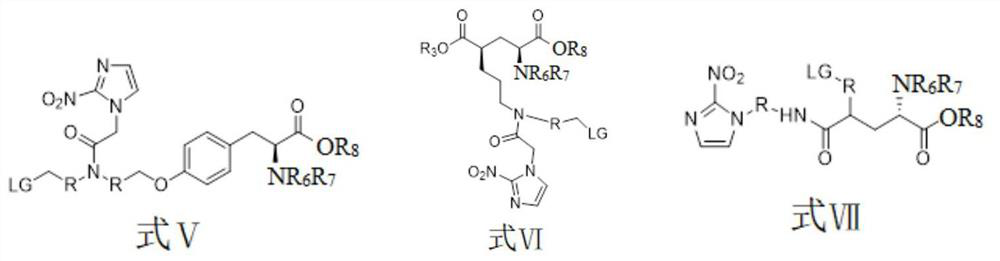
[0045] The preparation method of the compound shown in formula II is shown in reaction formula I:
[0046] The intermediate compound is obtained through fluorination by using a marked precursor compound of formula V containing an activating group, and then the compound of formula I is obtained through hydrolysis.
[0047]
[0048] The preparation method of the compound shown in formula III is shown in reaction formula II:
[0049] The intermediate compound is obtained through fluorination by using the marked precursor compound of formula VI containing an activating group, and then the compound of formula III is obtained through hydrolysis.
[0050]
[0051] The preparation method of the compound shown in formula IV is shown in reaction formula III:
[0052] Using a marked precursor compound of formula VII containing an activating group, the intermediate compound is obtained through fluorination, and then the compound of formula IV is obtained through hydrolysis.
[005...
Embodiment 1
[0060] Example 1 (S)-2-amino-3-(4-(2-(N-(3-fluoropropyl)-2-(2-nitro-1H-imidazol-1-yl)acetamido) Ethoxy)phenyl)propionic acid
[0061] The structural formula is as follows:
[0062]
[0063] The synthetic route is as follows:
[0064] 1) tert-butyl (2S)-3-(4-(2-(benzyl(3-((tetrahydro-2H-pyran-2-yl)oxy)propyl)amino)ethoxy)benzene base)-2-((tert-butoxycarbonyl)amino)propionate
[0065] Dissolve (S)-tert-butyl 2-((tert-butoxycarbonyl)amino)-3-(4-(2-(methylphenoxy)ethoxy)phenyl)propanoate (2 g, 3.74 mmol) in In 40mL of acetonitrile, N-benzyl-3-((tetrahydro-2H-pyran-2-yl)oxy)propane-1-amine (1.02g, 4.11mmol), sodium iodide (500mg, 2.24 mmol) and potassium carbonate (1.03g, 7.48mmol), reflux at 100°C for 28h. Then filter, and the filtrate is spin-dried to mix the sample, and the tert-butyl (2S)-3-(4-(2-(benzyl(3-( (tetrahydro-2H-pyran-2-yl)oxy)propyl)amino)ethoxy)phenyl)-2-((tert-butoxycarbonyl)amino)propionate (1.4 g, 70.0%) . 1 H NMR (300MHz, CDCl 3 )δ7.40–7.28(m,5H),7....
Embodiment 2
[0079] Example 2 (2S, 4R)-2-amino-4-(3-(N-(3-fluoropropyl)-2-(2-nitro-1H-imidazol-1-yl)acetamido)propane base) glutaric acid
[0080] The structural formula is as follows:
[0081]
[0082] The synthetic route is as follows:
[0083] 1) Di-tert-butyl (2S, 4R)-2-((tert-butoxycarbonyl)amino)-4-(3-(p-tolyloxy)propyl) glutarate
[0084] Dissolve di-tert-butyl (2S, 4R)-2-((tert-butoxycarbonyl)amino)-4-(3-hydroxypropyl) glutarate (900mg, 2.16mmol) in 25mL dichloromethane, ice Add p-toluenesulfonyl chloride (1.64g, 8.63mmol), triethylamine (6.67mL) and 4-dimethylaminopyridine (10mg, 0.08mmol) under bath conditions, react at room temperature for 12h, then wash with water three times, Na 2 SO 4 After drying, pass through flash chromatography (petroleum ether: ethyl acetate = 80:20) to obtain di-tert-butyl (2S, 4R)-2-((tert-butoxycarbonyl)amino)-4-(3-(para Tolyloxy)propyl)glutarate (790mg, 64.1%). 1 H NMR (300MHz, CDCl 3 )δ7.80(d, J=8.2Hz, 2H), 7.36(d, J=8.0Hz, 2H), 4.86(d, J=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com