A kind of l-aspartic acid-alpha-decarboxylase mutant and its application
A technology of aspartic acid and decarboxylase, applied in the field of genetic engineering, can solve problems such as less PanD, and achieve the effect of increasing self-shearing level and increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
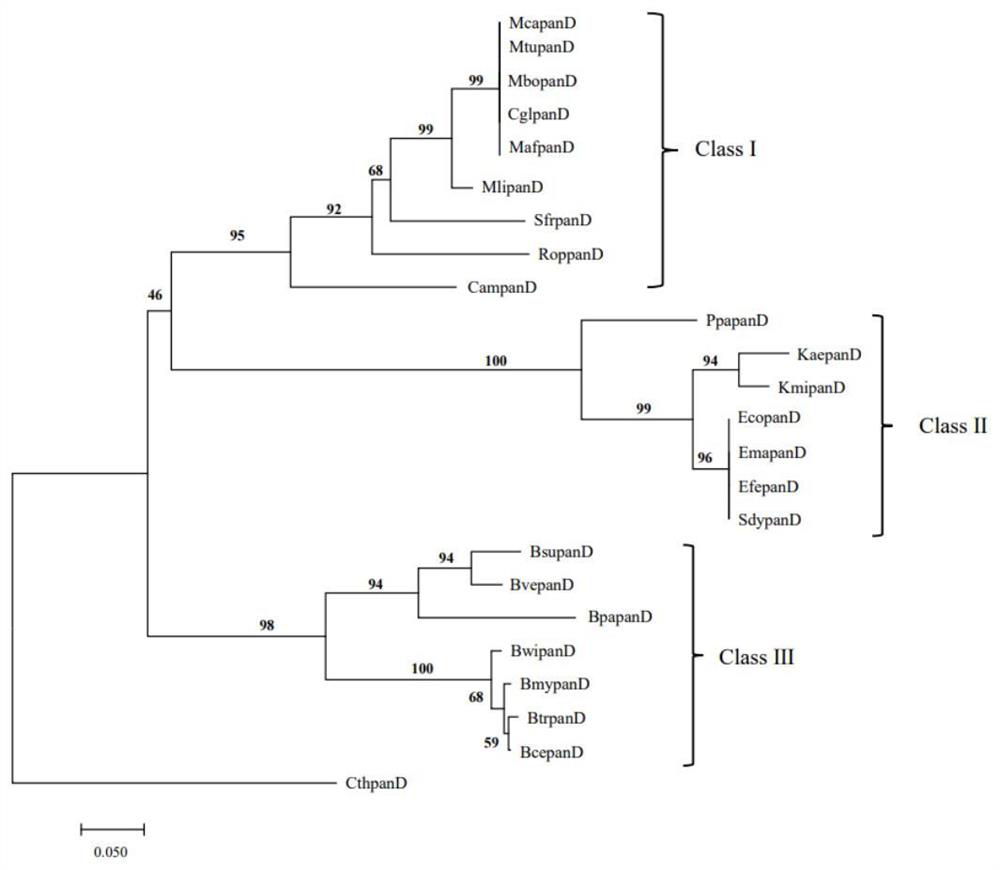
[0053] Example 1: Molecular evolution relationship analysis and selection pressure analysis of panD gene
[0054] (1) Molecular evolution relationship analysis of panD gene
[0055] This study explored the sequence differentiation of panD gene in different organisms by means of molecular evolution. Using the E. coli gene EcopanD (SAMN02604091) as the original template, a comprehensive search for the sequence of the panD gene family was performed through the NCBI website (https: / / www.ncbi.nlm.nih.gov / ). Confirmation criteria are that sequences with E value 40% are members of the panD gene family. The PanD amino acid sequences of 24 microorganisms were finally obtained, namely Mycobacterium tuberculosis (MtupanD), Mycobacterium africanum (MafpanD), Mycobacterium canettii (McapanD), Mycobacterium bovis (MbopanD), Bacillus cereus (BcepanD), Mycobacterium liflandii (MlipanD), Streptomycesfradiae ( SfrpanD), Rhodococcus opacus(RoppanD), Corynebacterium amycolatum(CampanD), Coryne...
Embodiment 2
[0063] Example 2: Construction of L-aspartate-α-decarboxylase mutants using whole plasmid site-directed mutagenesis
[0064] (1) Primers
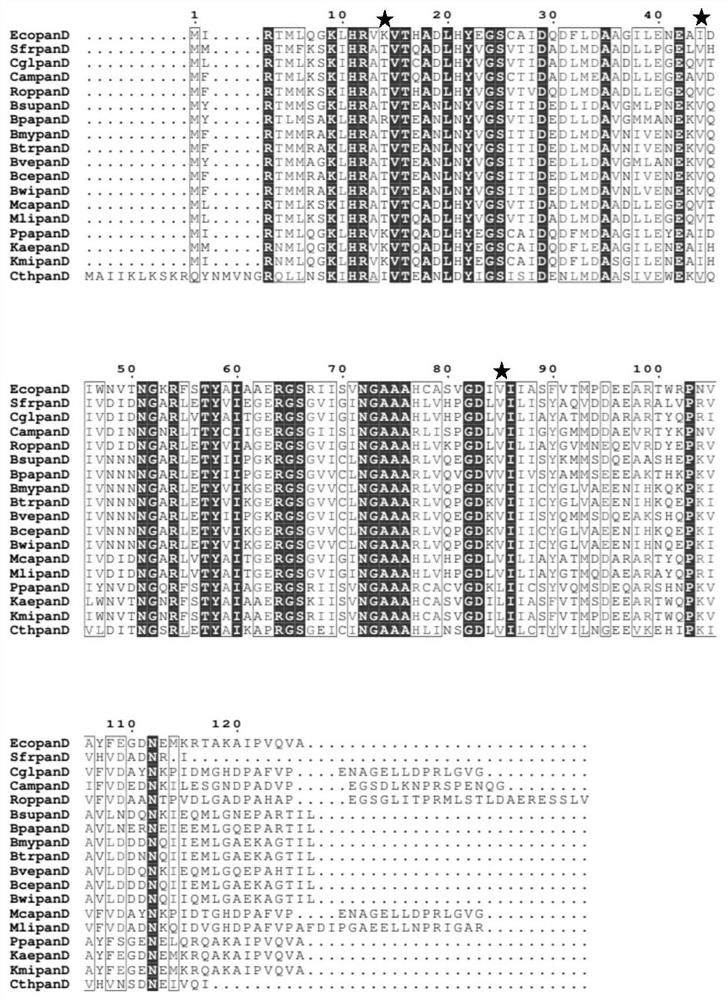
[0065] Further, based on the EcoPanD protein and the enantiomeric sites in Class III as the control, the above three sites were further subjected to directed evolution to form the L-aspartate-α-decarboxylase EcoPanD mutant library, which are as follows: (1) The lysine at position 14 of the amino acid shown in SEQ ID NO.1 was mutated to threonine (EcoPanD K14T , the nucleotide sequence of the mutant is shown in SEQ ID NO.2); (2) the 44th isoleucine of the amino acid shown in SEQ ID NO.1 is mutated into valine (EcoPanD I44V , the nucleotide sequence of the mutant is shown in SEQ ID NO.3); (3) the 85th amino acid shown in SEQ ID NO.1 is mutated from valine to leucine (EcoPanD V85L , the nucleotide sequence of the mutant is shown in SEQ ID NO.4). The PCR primer sequences used in this example are listed in Table 2. The EcopanD gene (derived f...
Embodiment 3
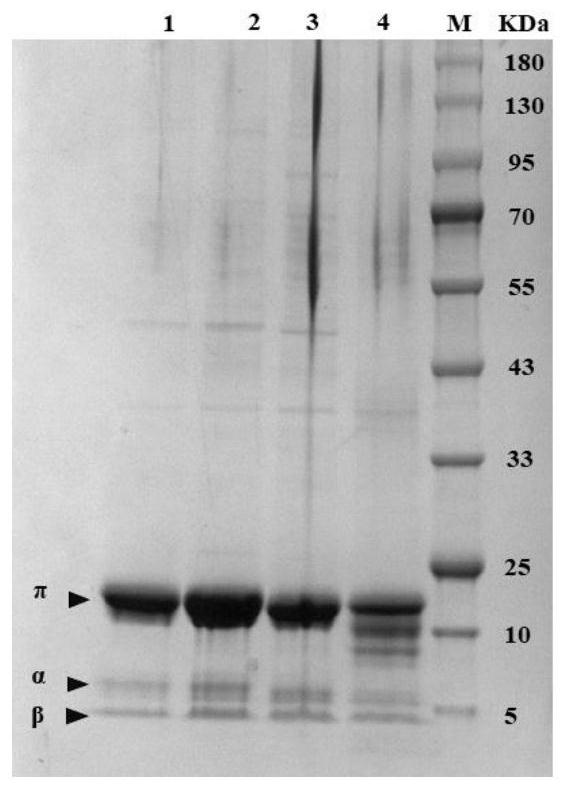
[0073] Example 3: Screening and Fermentation of Mutant Libraries
[0074] The transformants obtained in Example 2 were carried out as follows:
[0075] 1. Sequencing verification: Use sterilized pipette tips to select 3 transformants on the plate and inoculate them in LB liquid medium containing 100 μg / mL kanamycin, and at the same time, E.coli W3110 / pTrc99A-EcopanD was inoculated as a control in a 100 μg / mL Kana antibiotic in LB liquid medium. 37°C, 200r / min shaking culture for 12h, sequencing and bacteria preservation. The constructed strains are shown in Table 3.
[0076] Table 3 Strain constructed in Example 3
[0077]
[0078] 2. Induced expression of mutants: Under sterile conditions, take 1 mL of seed solution from the LB liquid medium in step 1, transfer it to 50 mL of LB medium (final concentration is 100 μg / mL Kana), 37 ° C, 200 r / Min shaking culture for about 2h until OD 600 0.6-0.8, add IPTG with a final concentration of 0.3mM, shake and culture for 12h fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com