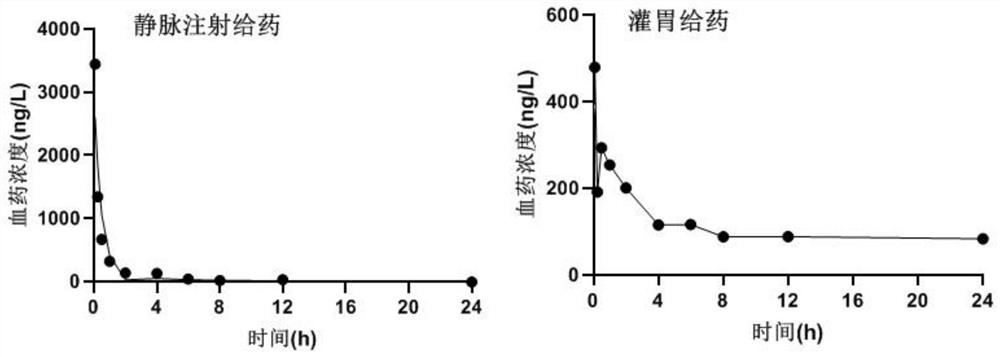
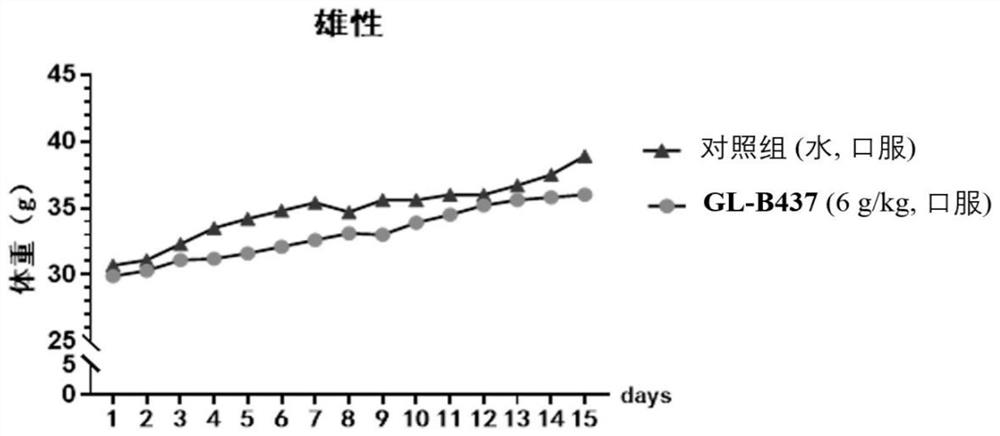
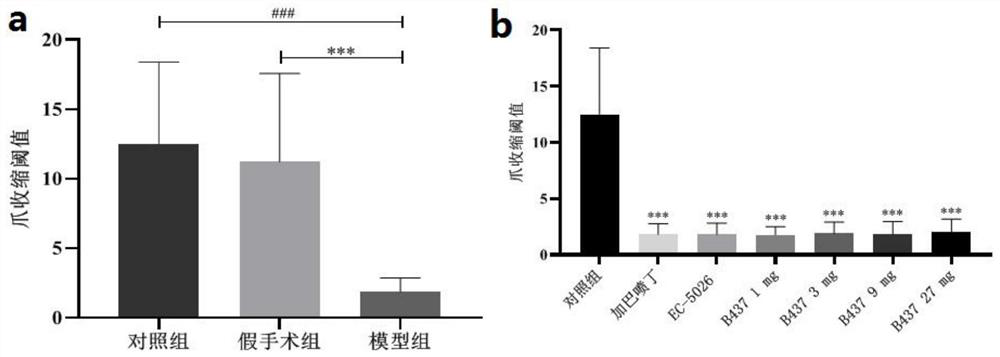
SEH inhibitor or pharmaceutically acceptable composition thereof as well as preparation method and application thereof
A technology of inhibitors and compositions, applied in the field of medicine, can solve the problems of short retention time of sEH inhibitors and poor efficacy in vivo, and achieve long efficacy in vivo, excellent therapeutic effect and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The present invention provides the preparation method of the sEH inhibitor described in the above technical scheme, comprising the following steps:
[0043] The compound II, the chlorination reagent, the catalyst and the soluble compound II solvent are mixed and then chlorinated to obtain an acid chloride intermediate; the acid chloride intermediate, ammonia solution, ice and the soluble acid chloride intermediate solution are mixed to carry out Acylation reaction to obtain the sEH inhibitor with the structure shown in formula I;
[0044]
[0045] In the present invention, unless otherwise specified, all raw material components are commercially available commodities well known to those skilled in the art.
[0046] In the present invention, the preparation method of compound II preferably comprises the following steps:
[0047] (1) compound 1, (S)-piperidine-3-carboxylic acid ethyl ester, organic base and soluble compound 1 solvent are mixed and then acylated to obtain...
Embodiment 1
[0091] (1) Preparation of (S)-ethyl 1-(3-fluoro-4-nitrobenzoyl)piperidine-3-carboxylate (compound 2)
[0092] Add 0.14mol 3-fluoro-4-nitrobenzoic acid, 150mL dry tetrahydrofuran and 0.014mol DMF to a 500mL single-neck flask to obtain a mixed solvent; 0.16mol thionyl chloride is dissolved in 50mL dry tetrahydrofuran to obtain chlorination Sulfoxide solution; add the thionyl chloride solution dropwise to the mixed solution under stirring conditions, after the dropwise addition, the temperature is raised to 60°C for chlorination reaction for 3 hours, and the tetrahydrofuran and the residual thionyl chloride are removed by distillation under reduced pressure to constant weight to obtain 3-fluoro-4-nitrobenzoyl chloride. Dissolve in 100 mL of dry tetrahydrofuran to obtain 3-fluoro-4-nitrobenzoyl chloride solution.
[0093] Under stirring conditions, 0.14mol (S)-piperidine-3-ethyl carboxylate, 0.41mol triethylamine and 150mL dry tetrahydrofuran were added to a 500mL three-necked fla...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com