Preparation and application of red light induced singlet oxygen response polymer nano anti-cancer drug
A technology of singlet oxygen and anticancer drugs, which is applied in the photodissociation of drugs in vivo, drug combination, antineoplastic drugs, etc., can solve the problems of monitoring, systemic toxic and side effects, lack of real-time imaging of drug enrichment, etc. Easy to obtain, reduce toxic and side effects, and avoid premature release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) will (10g, 5.0mmol) with (0.87g, 13mmol) was added to 30mL of toluene, then p-toluenesulfonic acid (0.22g, 1.3mmol) was added as a catalyst, and the water separator was refluxed for 48 hours at 135°C. After the reaction, the reaction solution was spin-dried with a rotary evaporator, and the crude product was co-precipitated with dichloromethane and ether to obtain a light yellow polymer (3.01g, 29%). 1 H NMR (400MHz, CDCl 3 )δ: 4.34(t, J=4.8Hz, 2H), 3.83-3.45(m, 174H), 3.38(s, 3H), 2.97(s, 1H);
[0034] (2) Will (2.01g, 0.97mmol) with (0.11g, 0.97mmol) was added to 10mL tetrahydrofuran, stirred at room temperature for 30min, and the tetrahydrofuran was spin-dried to obtain a light yellow polymer (2.06, 97%), 1 H NMR (400MHz, CDCl 3 )δ: 7.39(d, J=13.2Hz, 1H), 4.67(d, J=13.2Hz, 1H), 4.22(t, J=4.4Hz, 2H), 3.81(t, J=4.4Hz, 1H) ,3.74-3.44(m,176H),3.37(s,3H),2.96(br s,4H),1.84-1.70(m,5H);
[0035] (3) Will (0.50g, 0.23mmol) with (0.94g, 8.20mmol) was a...
Embodiment 2
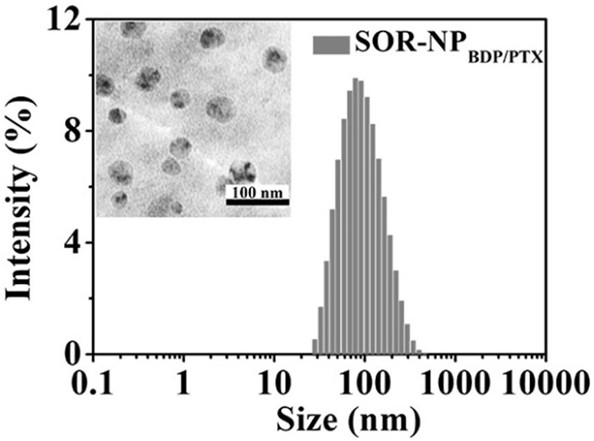
[0037] Red-light-induced singlet oxygen-responsive polymer nano-anticancer drug (SOR-NP BDP / PTX ) preparation: aminoacrylate bridged polyethylene glycol-polycaprolactone block copolymer SOR-mPEG-b-PCL (50 mg), BDP (2 mg) and PTX (2 mg) were dissolved in 1 mL of dichloromethane middle. A 1 wt % sodium cholate aqueous solution (2 mL) was added to the above solution. The mixture was emulsified by continuous ultrasonication for 4 min with a probe-type ultrasonic instrument under the condition of ice bath. Add 8 mL of newly prepared 0.5 wt % sodium cholate aqueous solution to the above emulsion, and stir overnight. After stirring, the volatile dichloromethane was removed with a rotary evaporator. Get stable nanoparticles SOR-NP BDP / PTX ,like figure 1 shown. SOR-NP BDP / PTX The hydrated particle size is about 84nm, and the particle size taken by the transmission electron microscope is about 44nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com