Olaparib dissolution enhancing composition
A composition and drug technology, applied in the field of olaparib dissolution enhancement composition, can solve problems such as limitations in the development of high-dose preparations, difficulty in swallowing for patients with advanced cancer, limited stability or solubilization ability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0060] Table 1
[0061]
[0062] Preparation method: Copovidone (PVP VA64, manufactured by BASF, Germany), dissolution accelerator (sulfobutyl-β-cyclodextrin (manufactured by Cyclolab Ltd., Hungary), hydroxypropyl-β-cyclodextrin (produced by Romano, France Gate company manufactures)), olaparib and colloidal silicon dioxide (Germany Evonik industrial group manufacture) are extruded with twin-screw extruder (screw diameter 11mm, ThermoScientific company) after mixing, obtain olaparib dissolution Enhanced composition.
[0063] Take the olaparib dissolution enhancing composition prepared in this example, after crushing, add the remaining excipients according to the prescription in Table 1 and mix evenly, and use a single-punch tablet press to form a preparation containing 150 mg of olaparib per tablet. Wherein sodium stearyl fumarate is manufactured by German JRS company), and PEG6000 is manufactured by U.S. Dow Chemical Company.
preparation Embodiment 2
[0065] Table 2
[0066]
[0067] Preparation method: dissolve copovidone (PVP VA64, manufactured by BASF, Germany), dissolution accelerators (sulfobutyl-β-cyclodextrin, hydroxypropyl-β-cyclodextrin) and olaparib in methanol / Acetone=1:4 solvent, the solvent was evaporated to obtain the olaparib dissolution enhancing composition.
[0068] Take the olaparib dissolution enhancing composition prepared in this example, after crushing, add the remaining excipients according to the prescription in Table 2 and mix evenly, and use a single-punch tablet machine to compress into a preparation containing 150 mg of olaparib per tablet.
preparation Embodiment 3
[0070] table 3
[0071]
[0072] Among them, sodium lauryl sulfate is manufactured by BASF of Germany, glyceryl behenate and labrasol are manufactured by Garfars of France, and Span 20 is manufactured by Nanjing Well Chemical Co., Ltd.
[0073] Preparation method: Copovidone, dissolution enhancer hydroxypropyl-β-cyclodextrin, olaparib, colloidal silicon dioxide, labrasol, sodium lauryl sulfate and Span 20 are mixed and extruded by twin-screw Machine extruded to obtain the olaparib dissolution enhancement composition.
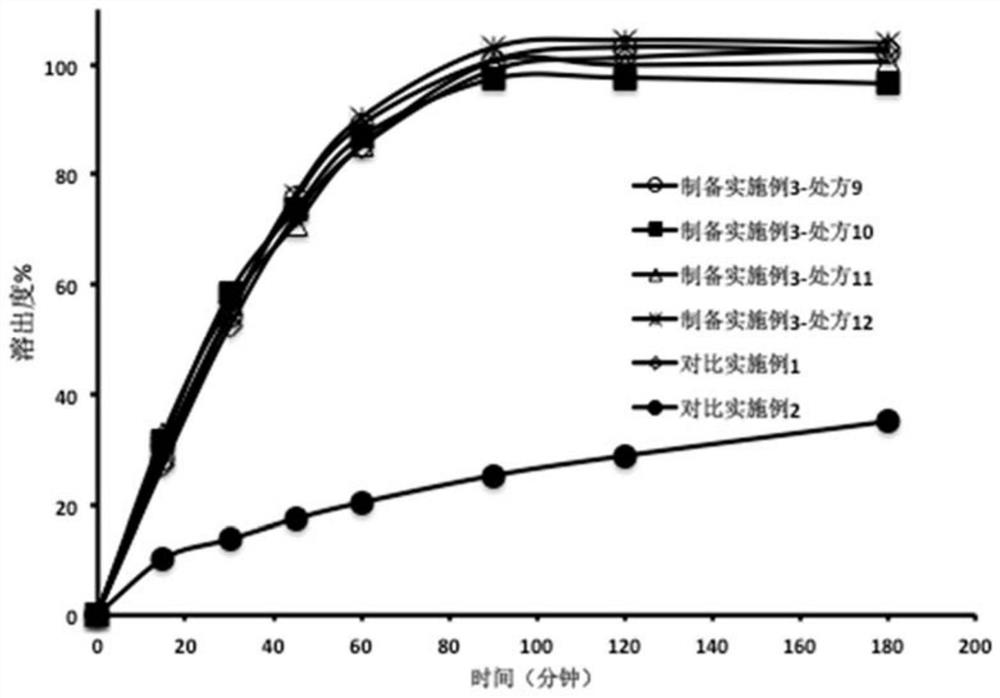
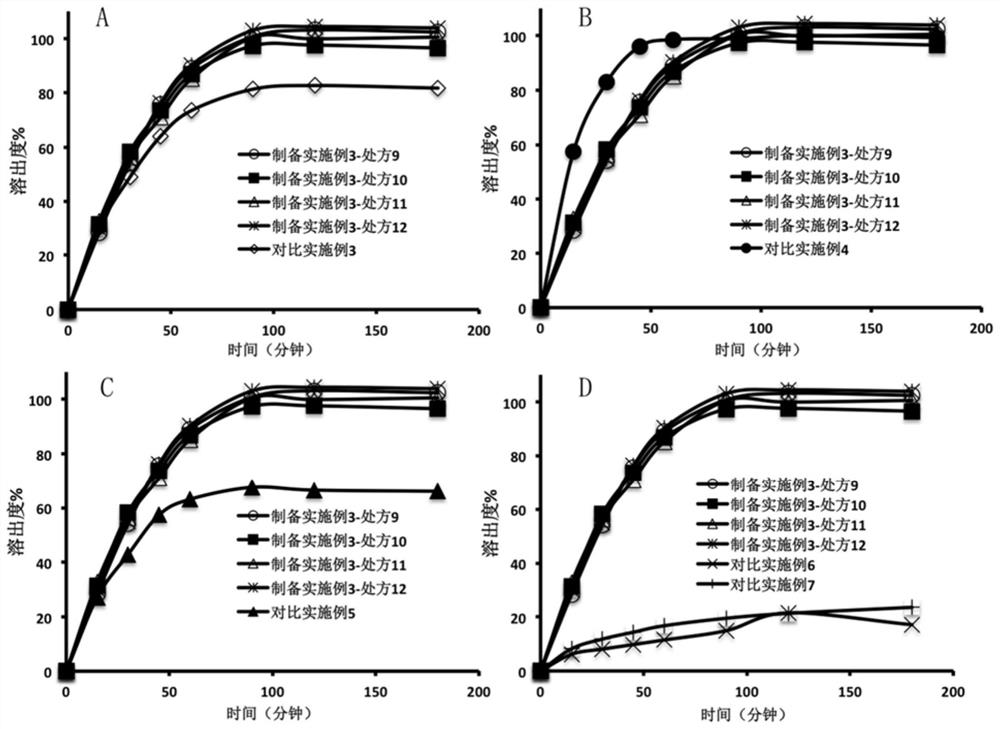
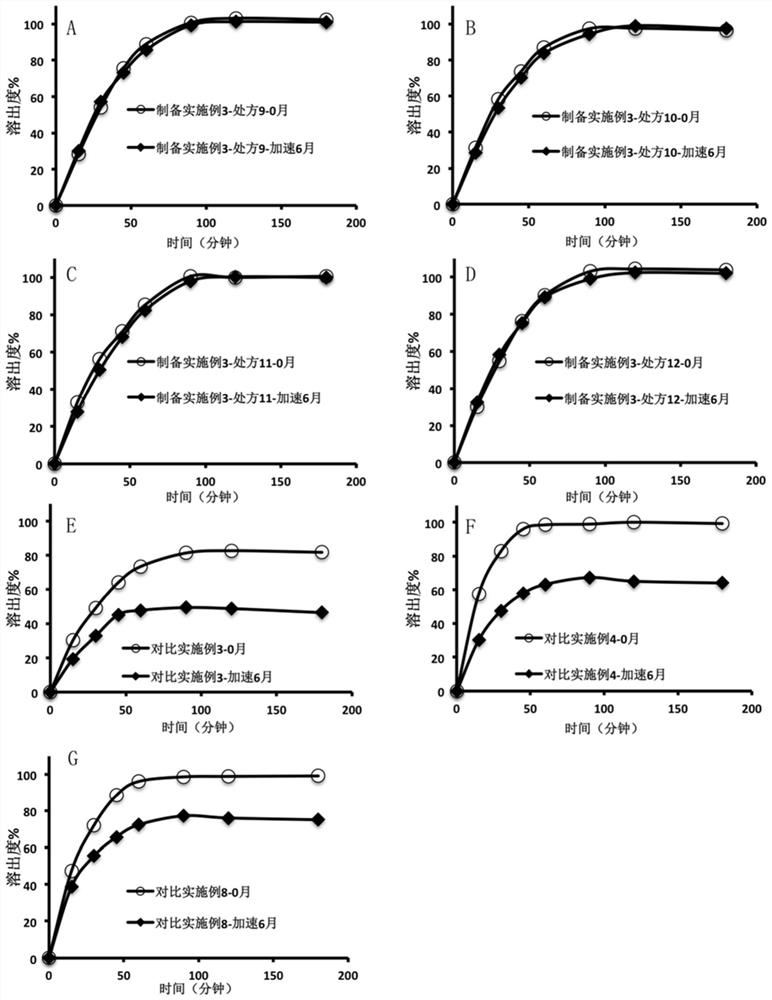
[0074] Take the olaparib dissolution enhancing composition prepared in this embodiment, after crushing, add the remaining excipients (sodium stearyl fumarate and glyceryl behenate) according to the prescription in Table 3 and mix evenly, and use a single-punch tablet press Compressed into a preparation containing 150 mg olaparib per tablet. Wherein, the tablet obtained in prescription 12 is taken, and then the tablet is placed in a coating pan, and the tabl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com