Application of medical composition in preparing medicine for treating xerophthalmia
A composition and dry eye technology, applied in the direction of medical preparations containing active ingredients, drug combinations, pharmaceutical formulas, etc., can solve problems such as premature cataract, side effects, glaucoma, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The composition of embodiment 1 pharmaceutical composition
[0033] First, the composition ratio of the medicinal composition is 37.5% of the wolfberry powder, 31.25% of the chrysanthemum powder, 2.5% of the blueberry powder, 2.5% of the grape seed powder, 0.25% of the zinc gluconate, 0.25% of the yeast selenium, The vitamin A contains 0.03%, the marigold powder 0.025% and the bitter tea oil are formulated according to the above ratio.
[0034] Preparation of wolfberry fruit powder (also chrysanthemum powder or blueberry powder or grape seed powder or marigold powder)
[0035] The wolfberry (also the chrysanthemum or the blueberry or the grape seed or the marigold) is dried and left to stand, and after cooling to room temperature, the wolfberry (also the chrysanthemum or the blueberry or the grape seed or the marigold) is pulverized to obtain wolfberry powder (also chrysanthemum powder or blueberry powder or grape seed powder or marigold powder).
[0036] The acquisit...
experiment example
[0044] Animal grouping and tube feeding
[0045] The present invention uses six-week-old ICR female mice as experimental animals, and divides ICR female mice into five groups. The grouping methods and experimental methods are as follows:
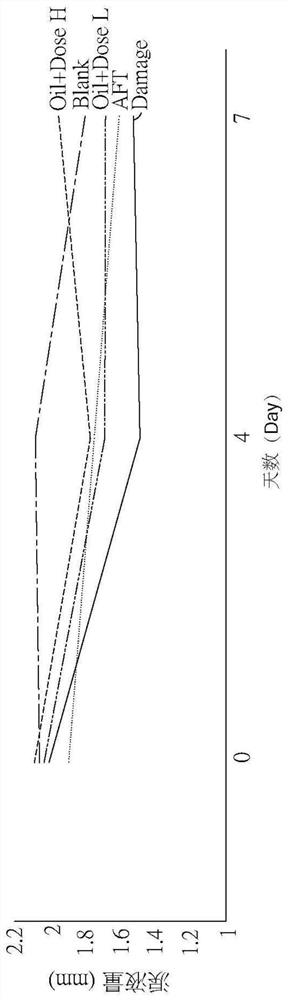
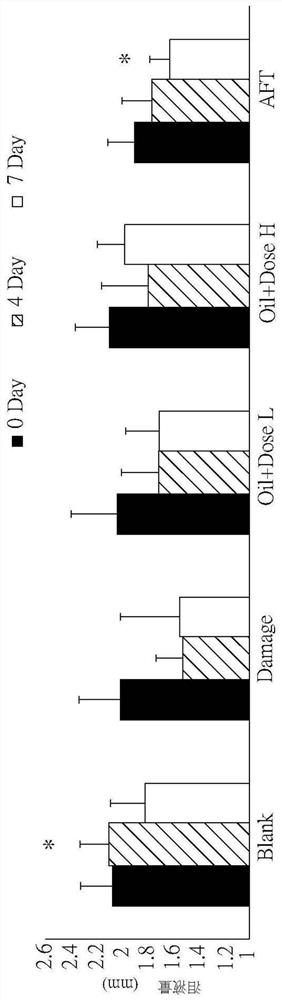
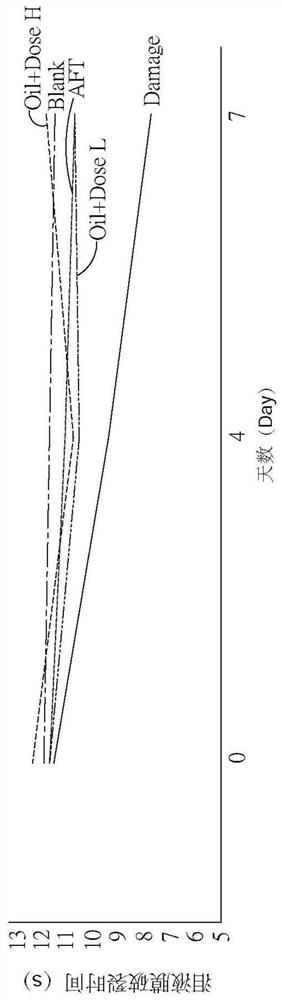
[0046] Please also refer to Figure 1A, which is a schematic diagram of tear volume according to an embodiment of the present invention, and Figure 1B, which is a schematic diagram of tear volume corresponding to different experimental conditions of eye axis distance according to an embodiment of the present invention, as shown in the figure In the figure, Blank is the above-mentioned normal control group, Damage is the injury control group, Oil+DoseL is the drug control group for dry eye syndrome using low doses of the pharmaceutical composition, and Oil+Dose H is the control group in the figure A drug control group for dry eye syndrome using high doses of the pharmaceutical composition, and AFT is a control group for using artificial tears....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com