Anti-oxidation bonding copper wire and preparation method thereof
A technology of bonding copper wires and oxidizing bonds, which is applied in the direction of coating, etc., can solve the problems of high oxidation characteristics of copper wires and inconvenient use, and achieve the effect of improving oxidation resistance and anti-oxidation performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
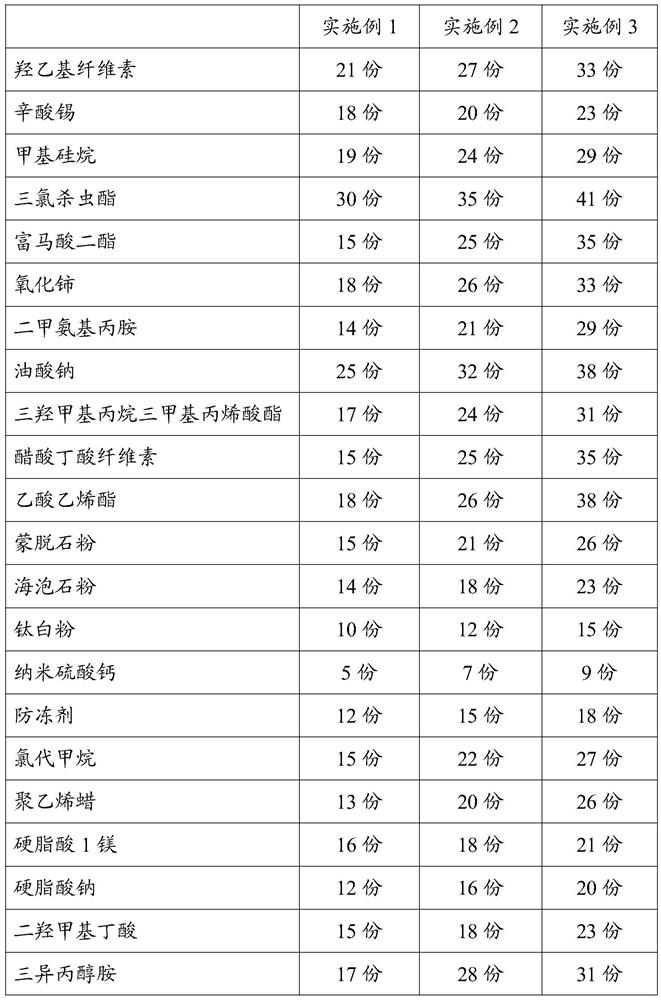
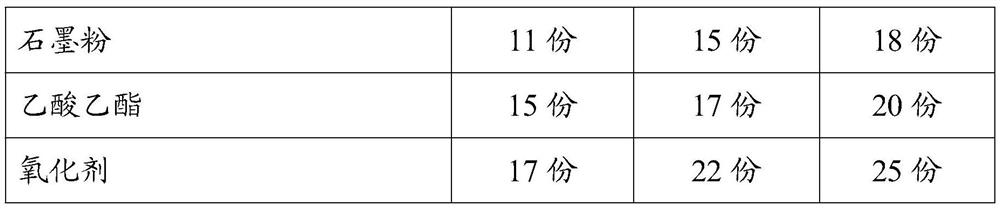
[0025] An anti-oxidation bonded copper wire, comprising a bonded copper wire and an anti-oxidation coating coated on the outer wall of the bonded copper wire, in parts by mass, the formula of the anti-oxidation coating includes: hydroxyethyl cellulose 21 parts, 18 parts of tin octoate, 19 parts of methyl silane, 30 parts of triclosinate, 15 parts of fumaric acid diester, 18 parts of cerium oxide, 14 parts of dimethylaminopropylamine, 25 parts of sodium oleate, trihydroxy 17 parts of methylpropane trimethacrylate, 15 parts of cellulose acetate butyrate, 18 parts of vinyl acetate, 15 parts of montmorillonite powder, 14 parts of sepiolite powder, 10 parts of titanium dioxide, 5 parts of nano calcium sulfate, antifreeze 12 parts, 15 parts of methyl chloride, 13 parts of polyethylene wax, 16 parts of magnesium stearate, 12 parts of sodium stearate, 15 parts of dimethylol butyric acid, 17 parts of triisopropanolamine, 11 parts of graphite powder , 15 parts of ethyl acetate, 17 parts...
Embodiment 2
[0038] The difference from Example 1 is that the formula of the anti-oxidation coating includes: 27 parts of hydroxyethyl cellulose, 20 parts of tin octoate, 24 parts of methyl silane, 35 parts of triclosulphate, fumaric acid diester 25 parts, 26 parts of cerium oxide, 21 parts of dimethylaminopropylamine, 32 parts of sodium oleate, 24 parts of trimethylolpropane trimethacrylate, 25 parts of cellulose acetate butyrate, 26 parts of vinyl acetate, montmorillonite 21 parts of stone powder, 18 parts of sepiolite powder, 12 parts of titanium dioxide, 7 parts of nano calcium sulfate, 15 parts of antifreeze, 22 parts of methyl chloride, 20 parts of polyethylene wax, 18 parts of magnesium stearate, 16 parts of sodium stearate 18 parts of dimethylol butyric acid, 28 parts of triisopropanolamine, 15 parts of graphite powder, 17 parts of ethyl acetate and 22 parts of oxidizing agent.
Embodiment 3
[0040]The difference from Example 1 is that the formula of the anti-oxidation coating includes: 33 parts of hydroxyethyl cellulose, 23 parts of tin octoate, 29 parts of methyl silane, 41 parts of triclosulphate, fumaric acid diester 35 parts, 33 parts of cerium oxide, 29 parts of dimethylaminopropylamine, 38 parts of sodium oleate, 31 parts of trimethylolpropane trimethacrylate, 35 parts of cellulose acetate butyrate, 38 parts of vinyl acetate, montmorillonite 26 parts of stone powder, 23 parts of sepiolite powder, 15 parts of titanium dioxide, 9 parts of nano calcium sulfate, 18 parts of antifreeze, 27 parts of methyl chloride, 26 parts of polyethylene wax, 21 parts of magnesium stearate, 20 parts of sodium stearate Parts, 23 parts of dimethylol butyric acid, 31 parts of triisopropanolamine, 18 parts of graphite powder, 20 parts of ethyl acetate, 25 parts of oxidizing agent. The specific formula data of three groups of embodiments of the present invention are as follows:
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com