Bithiophene-biscoumarin-based BODIPY near-infrared fluorescent dye and preparation method thereof
A technology of dicoumarin and fluorescent dyes, which is applied in the synthesis of organic compounds, functional fluorescent dyes and fine chemicals, can solve the problems of low selectivity, immature reaction, and many synthesis steps, and achieve high selectivity and synthetic methods Effect of mature, high molar extinction coefficient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The preparation of embodiment 1 dicoumarin base BODIPY derivative (II)
[0027] Under anhydrous conditions, in a dry round-bottomed flask equipped with a Dean-Stark apparatus, diiodo-BODIPY derivative (I) (618 mg, 1 mmol), 7-(N,N-diethylamino)coumarin -3-Formaldehyde (735mg, 3mmol) and p-toluenesulfonic acid (86mg, 0.5mmol) were dissolved in 25mL of toluene and 2mL of piperidine, heated to reflux at 125°C, and reacted for 6 hours. Cool to room temperature, extract with dichloromethane, wash with water, combine the organic layers, evaporate the organic solvent under reduced pressure, the residue is separated and purified by silica gel column chromatography, the eluent is 100% dichloromethane, and the gray-brown solid product 3 is obtained. 5-Dicoumarinyl BODIPY derivative (II) (375 mg, 35%). 1 HNMR (600MHz, CDCl 3 ): δ8.38-8.03(m, 2H), 8.00-7.86(m, 2H), 7.85-7.50(m, 2H), 7.41(t, J=8.4Hz, 2H), 6.99(d, J=9.0 Hz, 2H), 6.69(d, J=55.8Hz, 2H), 6.54(dd, J=19.8, 2.4Hz, 2H), 3...
Embodiment 2
[0028] The preparation of embodiment 2 bisthiophene-dicoumarin base BODIPY derivatives (III)
[0029] Under argon protection, 3,5-dicoumarin-based BODIPY derivative (II) (536 mg, 0.50 mmol), Pd(PPh 3 ) 4 (58mg, 0.05mmol), CuI (10mg, 0.05mmol) were dissolved in 30mL tetrahydrofuran and 10mL triethylamine, and then 2-ethynylthiophene (0.15mL, 1.5mmol) was added. The mixture was slowly heated to 60°C and stirred overnight. Cool to room temperature, extract with dichloromethane, wash with water, dry, evaporate the organic solvent under reduced pressure, the residue is separated and purified by silica gel column chromatography, the eluent is 100% dichloromethane, and the gray solid product dithiophene-bicoumarin is obtained Sulfosyl BODIPY derivative (III) (232mg, 45%). 1 H NMR (600MHz, CDCl 3 ): δ8.63(s, 2H), 8.05(s, 2H), 7.86-7.84(d, J=15.0Hz, 2H), 7.54(s, 2H), 7.43-7.42(d, J=6.0Hz, 2H), 7.29(s, 2H), 7.03(s, 2H), 7.00(s, 2H), 6.61(s, 2H), 6.49(s, 2H), 3.47(s, 8H), 2.38(s, 3H...
Embodiment 3
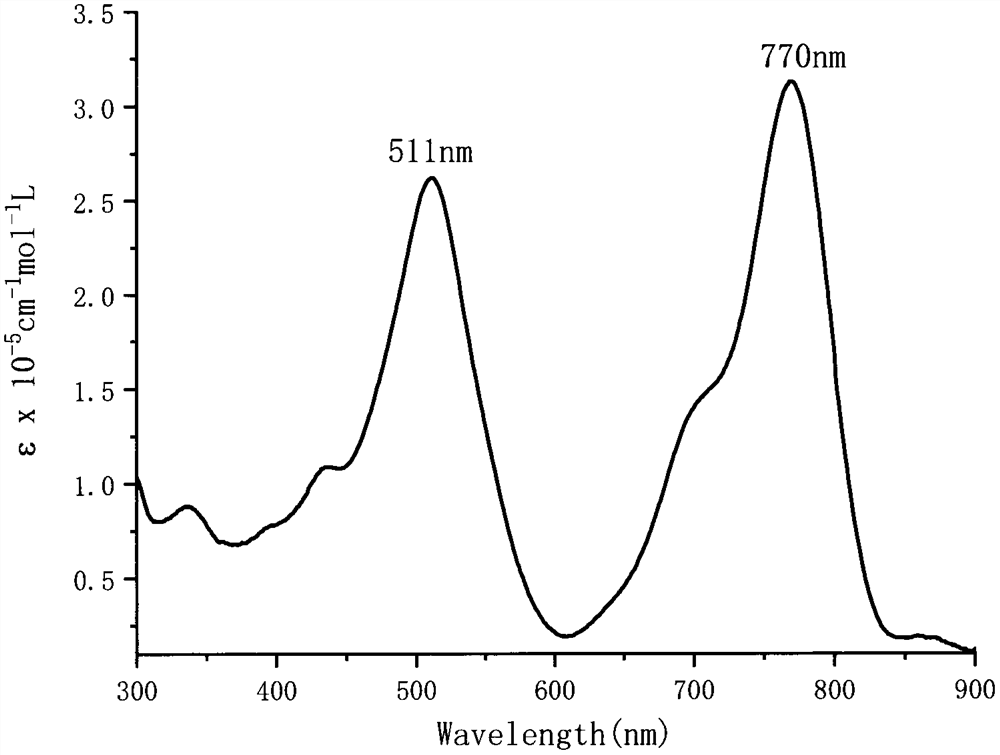
[0030]Example 3 Bithiophene-Dicoumarin-based BODIPY Derivatives (III) Solution UV-Vis Absorption Spectrum
[0031] Dissolve the bisthiophene-dicoumarin-based BODIPY derivative (III) in dichloromethane to a concentration of 1×10 - 5 mol / L dichloromethane solution, and measure its UV-Vis absorption spectrum. figure 1 The ultraviolet-visible absorption spectrum of the near-infrared fluorescent dye (III) solution prepared in Example 2 of the present invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com