Copper-chromium catalyst, preparation method thereof and method for preparing alkanol by olefine aldehyde or aldehyde hydrogenation
A catalyst, copper chromium technology, applied in the field of catalysis, can solve the problems of hidden safety hazards of production personnel, long process, large loss of metal salt raw materials, etc., and achieve the elimination of waste liquid containing metal ions, simple production process and high hydrogenation activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
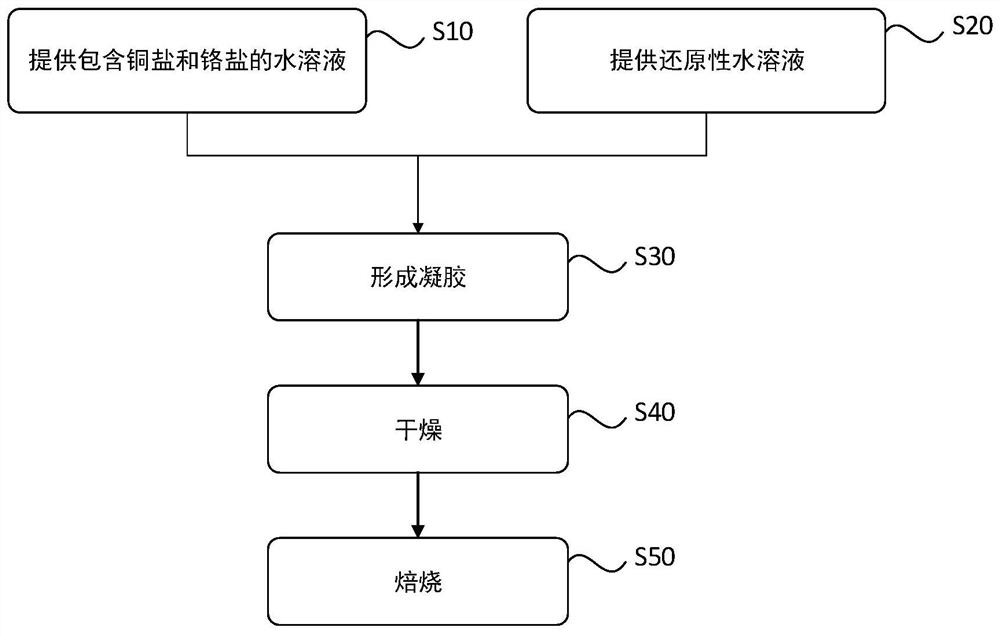
[0058] The inventors have provided an efficient and clean copper-chromium catalyst and a preparation method thereof through in-depth research on the nature of the active center of the copper-chromium catalyst and the reaction type in the catalyst preparation process. refer to figure 1 , the preparation method of a kind of copper chromium catalyst provided by one embodiment of the present invention comprises:
[0059] S10: providing an aqueous solution comprising copper salt and chromium salt;
[0060] S20: providing a reducing aqueous solution;
[0061] S30: forming a gel;
[0062] S40: drying;
[0063] S50: Roasting.
[0064] In the step S10 of providing the reaction solution, it includes: providing an aqueous solution comprising copper salt and chromium salt, wherein at least one of the copper salt and the chromium salt should use nitrate as an anion, for example, an aqueous solution of copper nitrate and chromium nitrate can be used as The reaction solution. The react...
Embodiment 1
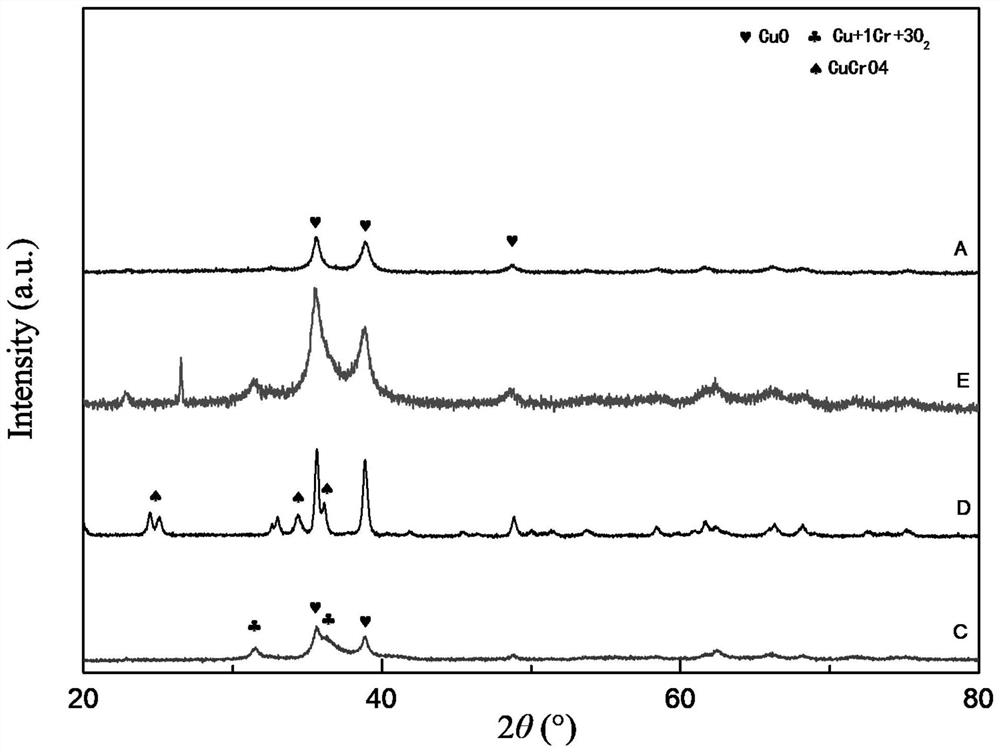
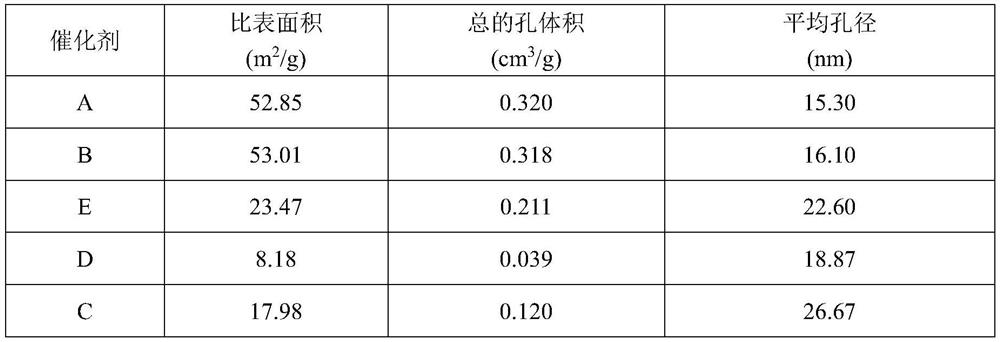
[0107] Measure 263.98mL of copper nitrate solution (concentration is 1.0mol / L) and 184.22mL of chromium nitrate solution (concentration is 1.0mol / L) respectively, and place them in a glass reactor with a stirring and heating jacket with a volume of 600mL. The ratio of copper to chromium is 1.43:1; start stirring and feed heat transfer oil, control the temperature in the reactor to 90°C; simultaneously add a total of 1186mL oxalic acid solution (concentration of 1.0mol / L) dropwise to the reactor , the molar ratio of oxalic acid to total nitrates was 1.1:1); the condensed collected evaporated water reached 1300mL and then stopped stirring to obtain 175g of gel. Gained gel is placed in the enamel dish, and is dried at 120 DEG C for 12 hours; The solid after drying is calcined at 300 DEG C for 4 hours, obtains the total 34.8g of black powder A, and the yield of metal ions is 99.42% (the yield of metal ions is according to Calculated by the following formula: mass of black powder A...
Embodiment 2
[0110] Measure 241.98mL of copper nitrate solution (concentration: 1.0mol / L) and 207.25mL of chromium nitrate solution (concentration: 1.0mol / L), and place them in a jacketed glass reactor with a volume of 600mL, in which copper and chromium The mass ratio is 1.17:1; start stirring and feed heat transfer oil, control the temperature in the reactor to 80°C; simultaneously add a total of 1106mL oxalic acid solution (concentration is 1.0mol / L, oxalic acid and total nitric acid The molar ratio of root is 1.0:1); Condensation collects evaporated water and stops stirring after reaching 1230mL, obtains 174g gel. The obtained gel was placed in an enamel dish and dried at 120° C. for 12 hours, and the dried solid was fired at 350° C. for 4 hours to obtain 34.9 g of black powder B (99.6% yield of metal ions). The black powder B was compressed into tablets by a tableting method, and the tableting pressure was 7.0 MPa to obtain catalyst B.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com