Benzylisoquinoline alkaloid with human carboxylesterase 2 inhibition effect and application thereof
A technology of benzyl isoquinoline and alkaloids, which is applied in the field of medicine and achieves the effects of high yield, conducive to sustainable industrial production and good research and development prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The preparation of Corydalis Corydalis extract, comprises the steps:
[0040] (1) Acetic acid processing: 50 kg of tubers of the traditional Chinese medicine Corydalis yanhusuo W.T.Wang were soaked in 10L of 6% acetic acid solution, and dried at 40°C.
[0041] (2) Extract: The medicinal material prepared with acetic acid was pulverized, soaked in water, and then extracted by ultrasonic for 3 times, each time with 50 L of water for 1 hour, combined the extracts, and concentrated under reduced pressure to obtain the plant extract.
Embodiment 2
[0043] The preparation of benzylisoquinoline alkaloid compound comprises the steps:
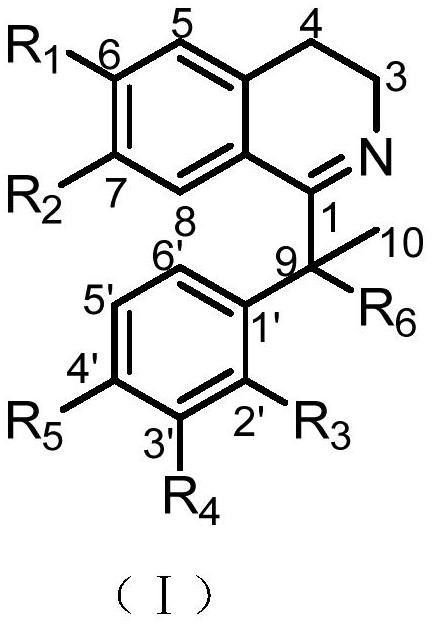
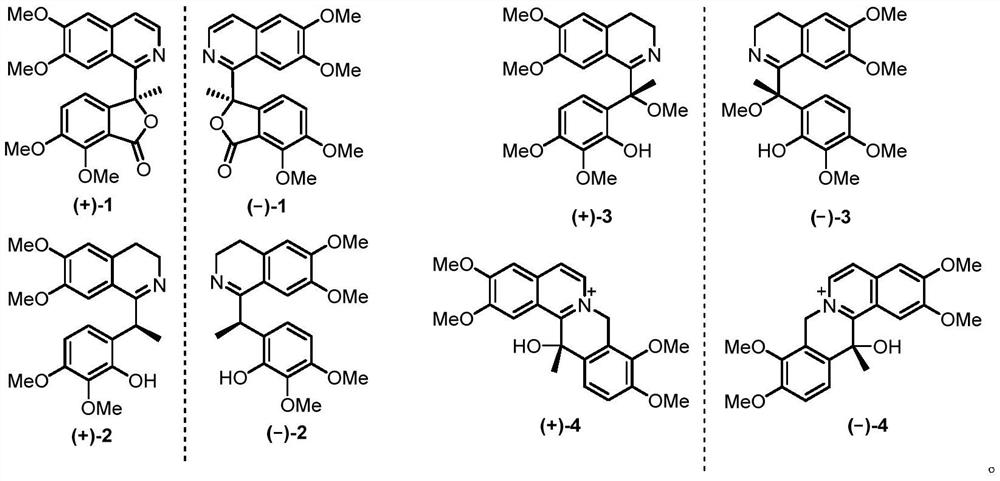
[0044] The extract obtained in Example 1 was subjected to macroporous adsorption resin column chromatography, and eluted successively with water, 50% ethanol, and 95% ethanol respectively, and the eluent was concentrated under reduced pressure; the 95% ethanol extract was concentrated to no alcohol, and distilled water was added 2L, extracted 5 times with an equal volume of ethyl acetate to obtain an ethyl acetate phase and an aqueous phase. Ethyl acetate part E was obtained after recovering the organic solvent under reduced pressure. Sample E was eluted by silica gel column chromatography (dichloromethane-methanol 200:1, 150:1, 100:1, 50:1, 25:1, 10:1, 5:1, 3:1, 0:1) , to get component A-I. Component A was selected and then eluted by silica gel column chromatography (petroleum ether-acetone 30:1, 15:1, 10:1, 5:1, 3:1, 2:1, 1:1, acetone) to obtain the component A1-A8. Component A4 was sub...
experiment example 1
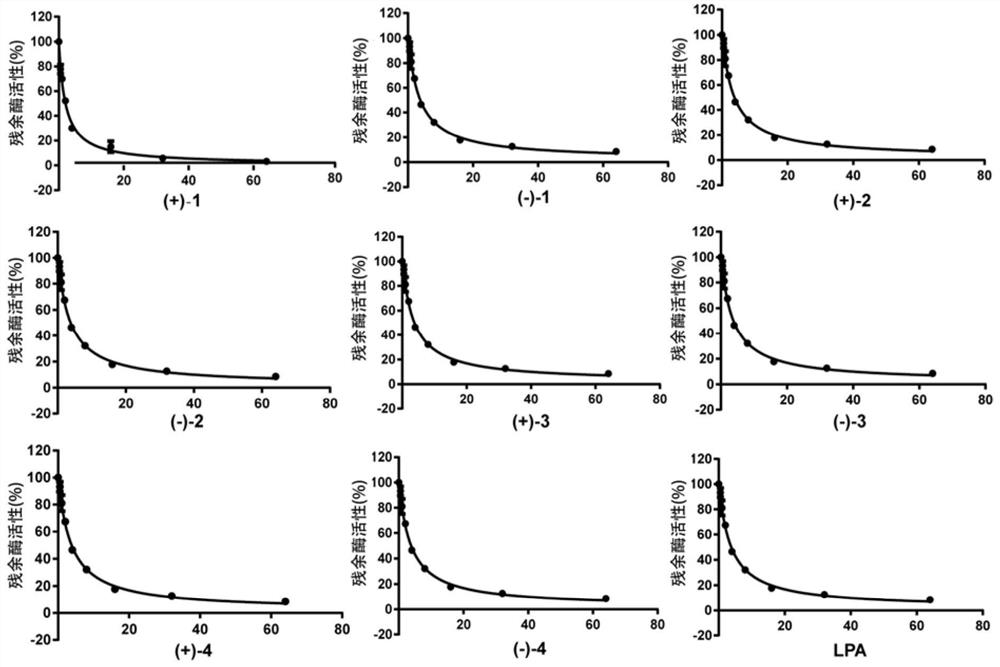
[0056] Experimental example 1: Evaluation of the inhibitory effect of the extract on hCE2 activity
[0057] Instruments: metal incubator, vortex shaker, microplate reader
[0058] Reagents: including 0.5mM substrate FD (diacetylfluorescein), 0.1mM phosphate buffer saline PBS (pH7.4), ACN (acetonitrile), HLM human liver microsomes / single enzyme
[0059] Experimental steps:
[0060] 1) The microsome incubation system contains the following components (total volume 200μl):
[0061]
[0062] Table 3. hCE-2 incubation system and dosage
[0063]
[0064] 2) Add HLM+PBS+Inhibitor / DMSO to a black 96-well plate, shake and mix on a vortexer, and pre-incubate at 37°C for 10 minutes;
[0065] 3) After adding FD to start the reaction, perform fluorescence analysis (fluorescence microplate reader);
[0066] 4) set up three control groups simultaneously, one is no inhibitor (add equal volume DMSO) group as the 100% contrast of enzyme activity, the second is no enzyme (add equal vol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com