Method for producing 1, 4-butanediamine by using recombinant escherichia coli
A technology for recombining Escherichia coli and butanediamine, applied in the field of genetic engineering, can solve the problems of long transformation period, low yield, low yield and the like, and achieve the effect of improving specific enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment 1: Construction of recombinant bacteria Escherichia coli BL21 / pXMJ19-speA-speB, Escherichia coliBL21 / pXMJ19-A533P-speB, Escherichia coli BL21 / pXMJ19-D531R-speB and Escherichiacoli BL21 / pXMJ19-I534G-speB
[0064] (1) According to the gene sequence of speA (nucleotide sequence shown in SEQ ID NO.2) and speB (nucleotide sequence shown in SEQ ID NO. 4), design the PCR primer F of arginine decarboxylase gene 1 and R 1 , PCR primer F of agmatine enzyme gene 2 and R 2 .
[0065] f 1 : 5'-ggtcgactctagaggatccaaaggaggaaaatcatgtctgacgacatgtctatggg-3'
[0066] R 1 : 5'-ttccacacattatacgagccgatgattaattgtcaagaattcttactcatcttcaagataagtataaccg-3'
[0067] f 2 : 5'-gtataatgtgtggaattgtgagcggataacaaaaaaggaggacaaccatgagcaccttaggtcatcaatac-3'
[0068] R 2 : 5'-gtatcaggctgaaaatcttctctcatccgaattcttactcgccctttttcgccgcctg-3'
[0069] (2) Cloning of arginine decarboxylase gene and agmatine enzyme gene
[0070] Using the total DNA of Escherichia coli str.K-12substr.MG1655 as...
Embodiment 2
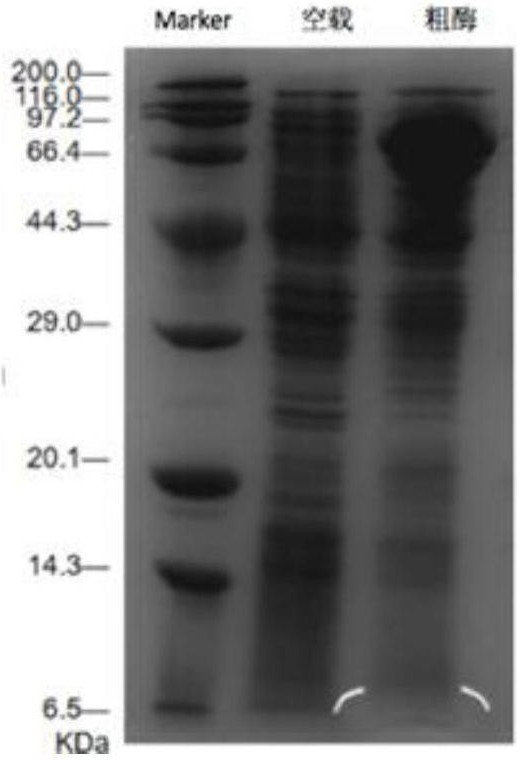
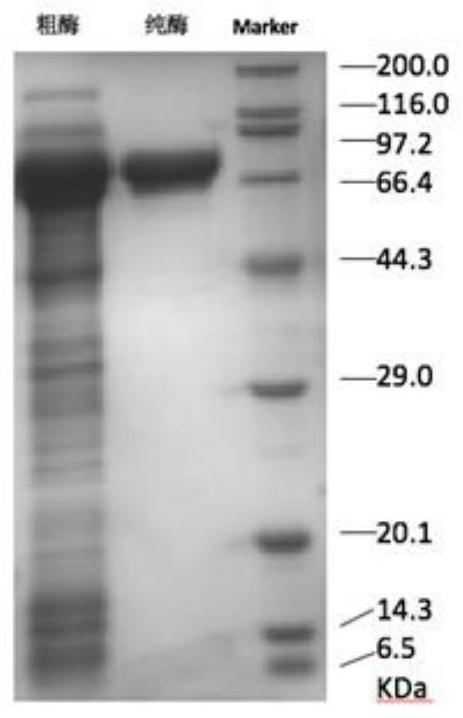
[0092] Embodiment 2: the specific enzyme activity determination of arginine decarboxylase mutant
[0093] Specific steps are as follows:
[0094] (1) Obtaining wild-type arginine decarboxylase and mutant A533P, D531R and I534G crude enzyme solutions
[0095] The recombinant E. coli Escherichia coli BL21 / pXMJ19-speA-speB, Escherichia coli BL21 / pXMJ19-A533P-speB, Escherichia coli BL21 / pXMJ19-D531R-speB and Escherichia coli BL21 / pXMJ19-I534G-speB prepared in Example 1 were respectively prepared Added at a concentration of 10 μg·mL -1 Streak culture on the chloramphenicol-resistant LB solid medium plate, pick a single colony and inoculate them to a concentration of 10 μg·mL -1 In chloramphenicol-resistant 10 mL LB liquid medium, shake the flask for 12 hours at a temperature of 37° C. and 200 rpm to obtain a seed solution.
[0096] The seed solution was inoculated with an inoculation volume of 1% (v / v) to a concentration of 10 μg mL -1 In chloramphenicol-resistant 50mLLB liquid...
Embodiment 3
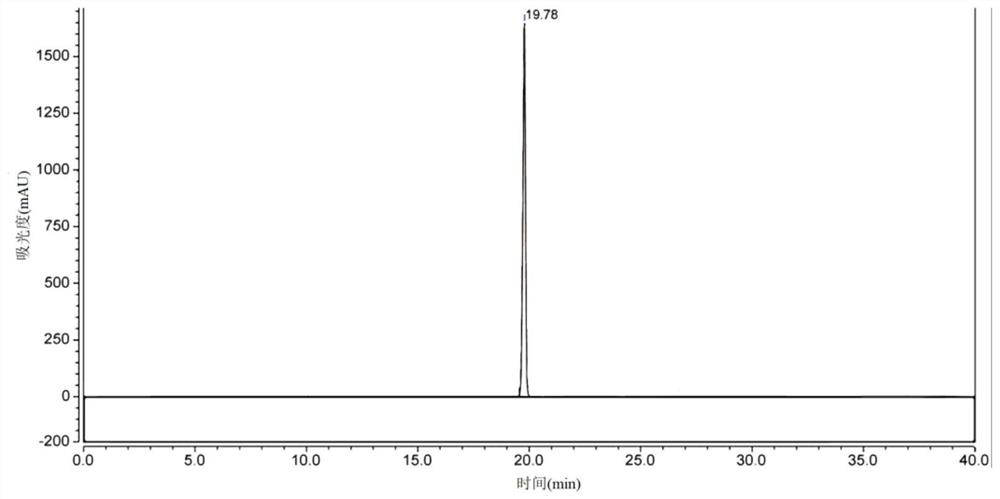
[0106] Example 3: Production of 1,4-butanediamine by recombinant Escherichia coli whole cells
[0107] Specific steps are as follows:
[0108] (1) The recombinant bacteria Escherichia coli BL21 / pXMJ19-speA-speB, Escherichia coli BL21 / pXMJ19-A533P-speB, Escherichia coli BL21 / pXMJ19-D531R-speB and Escherichia coli BL21 / pXMJ19-I534G- After speB was activated by streaking on the LB solid plate, pick a single colony and inoculate it in 10 mL with a concentration of 10 μg·mL -1 In the chloramphenicol-resistant LB liquid medium, shake the flask for 12 hours under the conditions of 37 ° C and 200 rpm to obtain the seed solution;
[0109] (2) Transfer the prepared seed solution to 200mL TB liquid medium with an inoculum size of 1% (v / v), culture it at 37°C and 200rpm for 2 hours, and then add IPTG with a final concentration of 0.5mM. Low-temperature induction at 16°C and 200rpm for 12 hours to obtain a culture medium;
[0110](3) The culture solution was centrifuged at 8000 rpm and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com