Dopamine functionalized poly(beta-amino ester) as well as preparation method and application thereof
A dopamine and functionalized technology, applied in the biological field, can solve the problems of insufficient intracellular release and increased cytotoxicity, and achieve the effects of improving stability, low cytotoxicity and high transfection efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
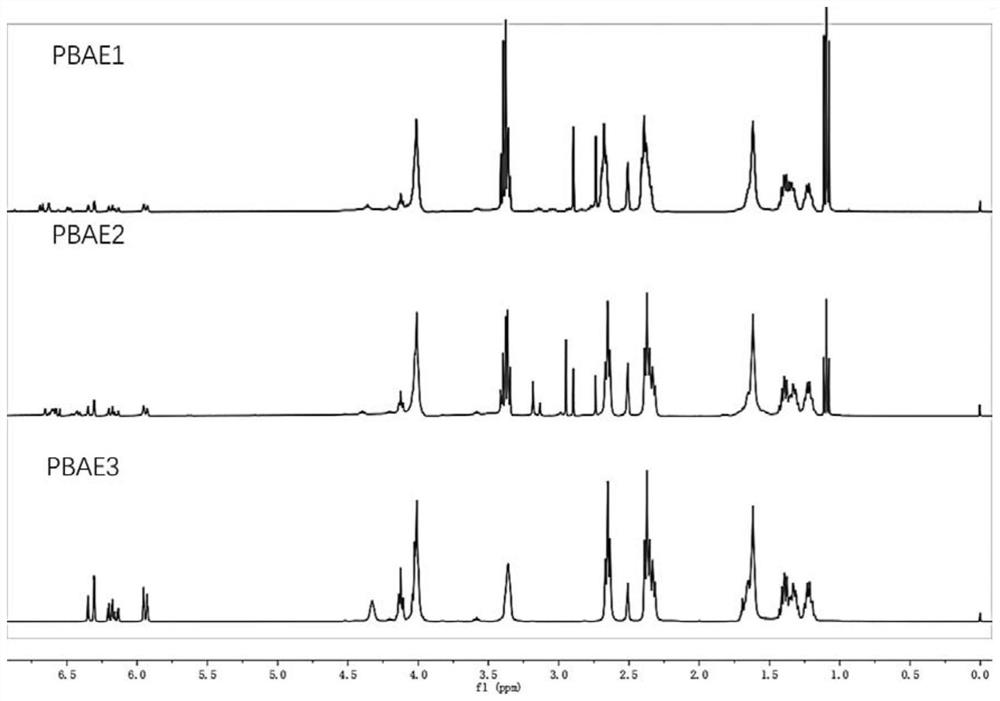
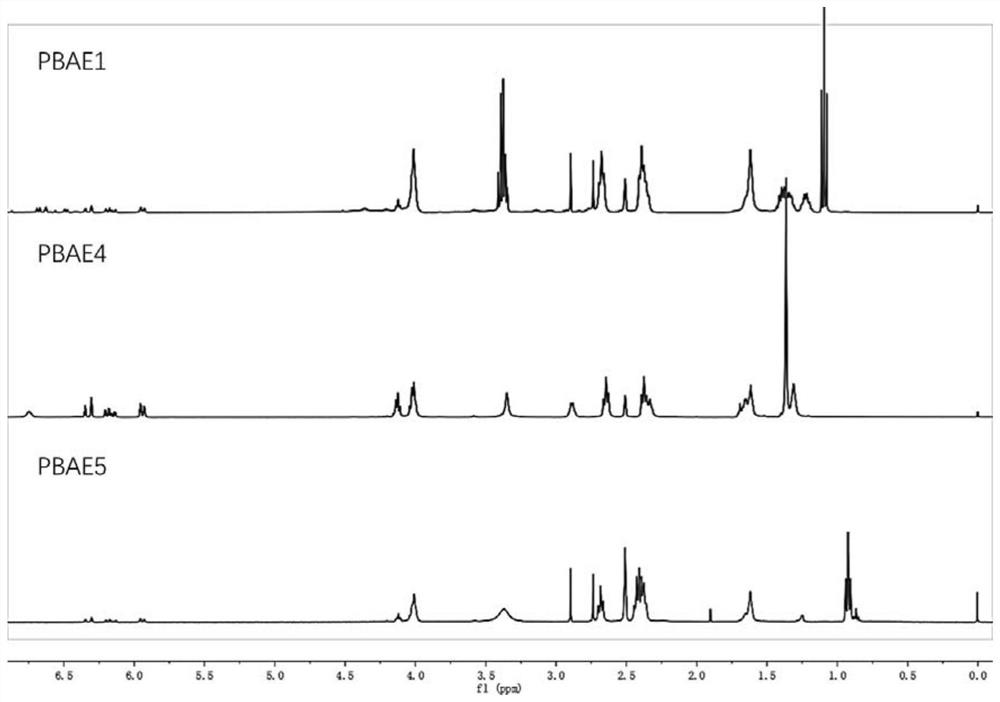
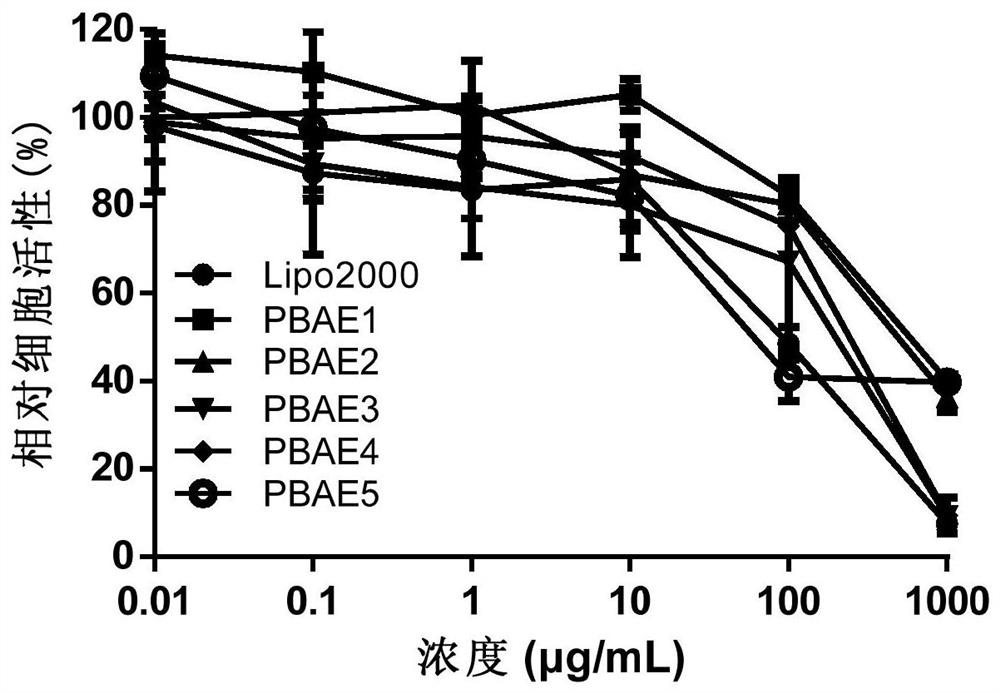
Embodiment 1
[0040] (1) Hydroxyl protection of dopamine phenol: dissolve dopamine hydrochloride (2mmol) and imidazole (2.4mmol) in dichloromethane solution (10mL), add dimethyl tert-butylchlorosilane (4mmol) dropwise in ice bath , The reaction was stirred at 25°C for 12h. After the reaction, the mixed solution is rotary evaporated to obtain a crude product, and the dopamine monomer whose phenolic hydroxyl group is protected is obtained by solution washing, liquid separation, rotary evaporation, and drying;
[0041] (2) After mixing the dopamine monomer (0.5mmol) prepared in step (1) with 5-amino-1-pentanol (2mmol), together with 1,4-butanediol diacrylate (2.75mmol) in di Stirring and reacting in methyl sulfoxide at 90°C for 24 hours to obtain a polymer;
[0042] (3) Dissolving the polymer (1 mmol) prepared in step (2) in tetrahydrofuran solution (4 mL), adding 2 mmol N-aminopropylmorpholine to the reaction system, and stirring at room temperature for 4 h to obtain a capped polymer;
[00...
Embodiment 2~3
[0045] According to the preparation process of Example 1, the amounts of dopamine monomer and 5-amino-1-pentanol in step (2) were replaced by 1mmol and 1.5mmol, 1.25mmol and 1.25mmol respectively, and the remaining steps were unchanged to obtain dopamine respectively. Functionalized poly(β-amino ester) PBAE2 and PBAE3.
Embodiment 4~5
[0047] According to the preparation process of Example 1, the 5-amino-1-pentanol in step (1) is replaced by N,N-dimethylethylenediamine and N,N-diethylethylenediamine respectively, and the remaining steps are not Changes to obtain dopamine-functionalized poly(β-amino ester) PBAE4 and PBAE5, respectively.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com