Hemotrophin and chorionic gonadotrophin composite hormone freeze-dried powder and preparation method thereof
A chorionic gonadotropin and freeze-dried powder technology, which can be used in freeze-dried transportation, sexual diseases, powder transportation, etc., can solve problems such as uneven effects, achieve improved effect, no side effects and safety hazards, and maintain biological activity. and stability effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
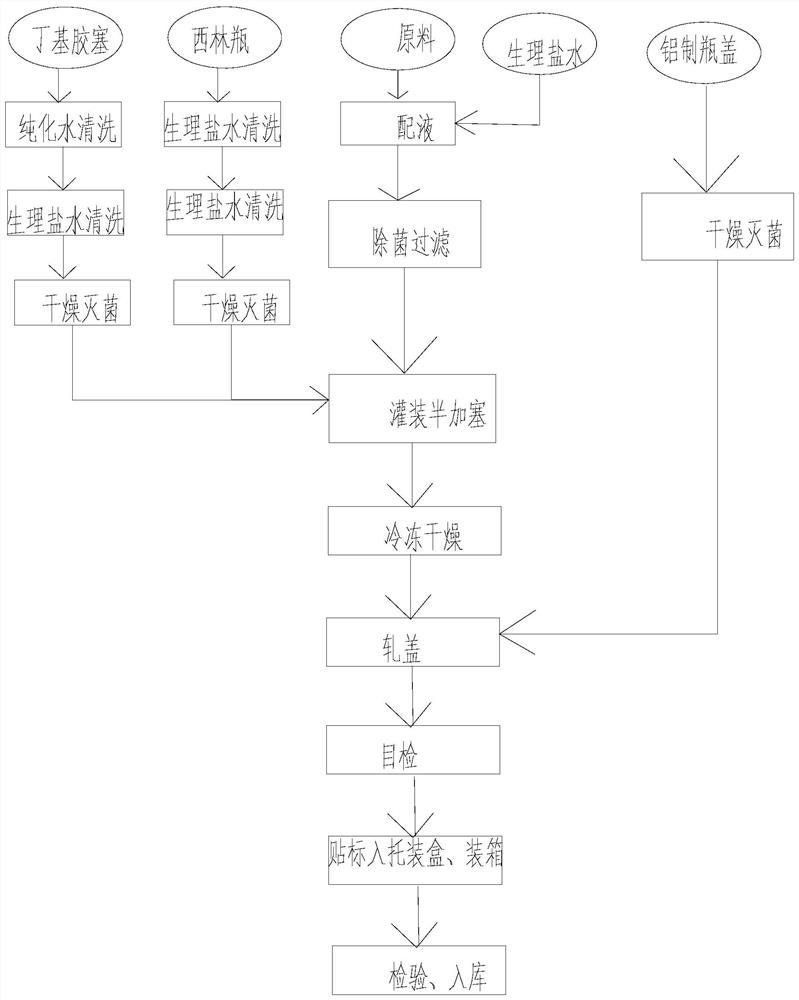
[0037] The second aspect of the present invention provides a kind of preparation method of described blood gonadotropin and chorionic gonadotropin composite hormone freeze-dried powder, at least comprising the following steps:
[0038] (1) In a sterile environment, add gonadotropin and chorionic gonadotropin into water at 0-40°C, and stir to obtain mixture A;
[0039] (2) In a sterile environment, add the excipients to the mixture A, and stir to obtain the mixture B;
[0040] (3) In a sterile environment, add a buffer to the mixture B to obtain a mixture C, the pH of the mixture C is 6.8-7.3;
[0041] (4) The mixture C is sterilized and filtered, and then filled, and the product D is obtained after the filling is completed, and the product D is freeze-dried and capped to obtain a packaged compound hormone freeze-dried powder.
[0042] As a preferred technical scheme, the freeze-drying at least includes the following steps:
[0043] (1) Put the product D into a freeze-drying ...
Embodiment 1
[0048] A blood gonadotropin and chorionic gonadotropin composite hormone freeze-dried powder in this embodiment, the preparation raw materials of every 1g blood gonadotropin and chorionic gonadotropin composite hormone freeze-dried powder include: 300IU blood gonadotropin, 100IU chorionic gonadotropin, 20mg stabilizer, 100mg excipient, buffer and water, the chorionic gonadotropin was purchased from Ningbo Sansheng Pharmaceutical Co., Ltd., and the chorionic gonadotropin was purchased from Shandong Xinkang Biotechnology Co., Ltd. .
[0049]The stabilizer is lecithin, and the lecithin is purchased from West Asia Reagent, CAS No.: 8002-43-5.
[0050] The excipient is a mixture of mannitol and α-lactose, the mass ratio of mannitol and α-lactose is 3:1, the mannitol is purchased from Aladdin reagent, CAS number: 69-65-8, the α-Lactose was purchased from West Asia Reagents, CAS No.: 5989-81-1.
[0051] The buffer is a mixture of citric acid and sodium salt, the mass ratio of citri...
Embodiment 2
[0063] A blood gonadotropin and chorionic gonadotropin composite hormone freeze-dried powder in this embodiment, the preparation raw materials of every 1g blood gonadotropin and chorionic gonadotropin composite hormone freeze-dried powder include: 300IU blood gonadotropin, 100IU chorionic gonadotropin, 20mg stabilizer, 100mg excipient, buffer and water, the chorionic gonadotropin was purchased from Ningbo Sansheng Pharmaceutical Co., Ltd., and the chorionic gonadotropin was purchased from Shandong Xinkang Biotechnology Co., Ltd. .
[0064] The stabilizer is lecithin, and the lecithin is purchased from West Asia Reagent, CAS No.: 8002-43-5.
[0065] The excipient is a mixture of mannitol and α-lactose, the mass ratio of mannitol and α-lactose is 4:1, the mannitol is purchased from Aladdin reagent, CAS No.: 69-65-8, the α-Lactose was purchased from West Asia Reagents, CAS No.: 5989-81-1.
[0066] The buffer is a mixture of citric acid and sodium salt, the mass ratio of citric ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com