Gastrodin derivatives as well as preparation method and application thereof
A technology of gastrodin and derivatives, applied in the field of medicine, to achieve the effects of sedative and analgesic brain circulation, inhibition of nerve cell damage, and inhibition of activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
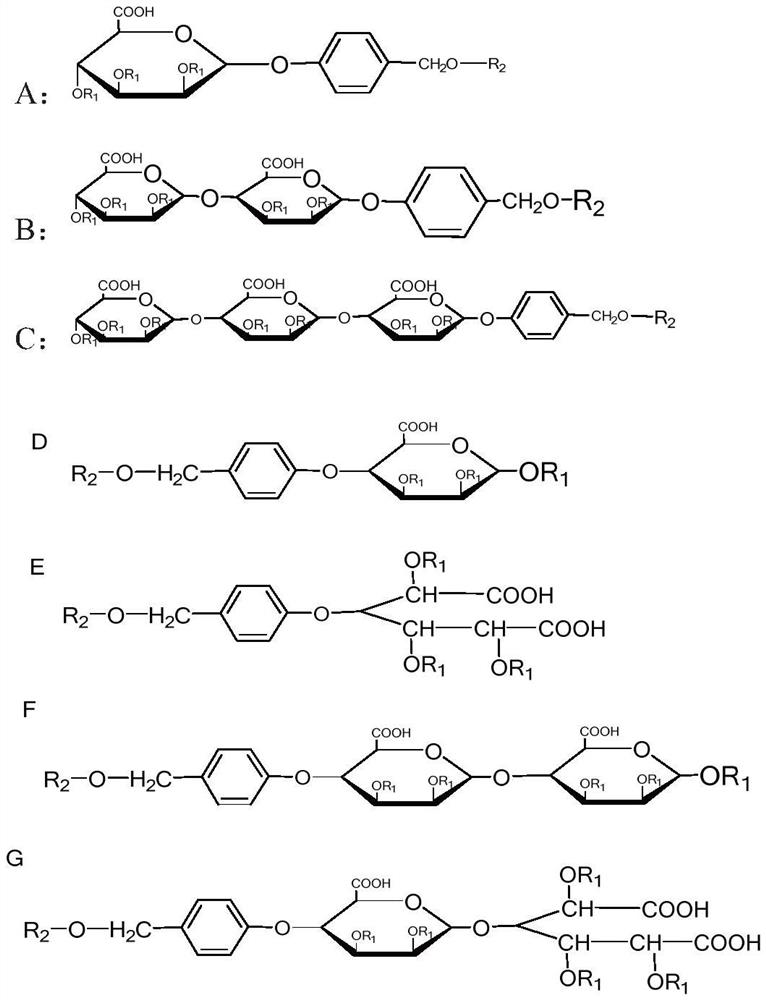
[0033] Embodiment 1, the preparation method of series compound A-G
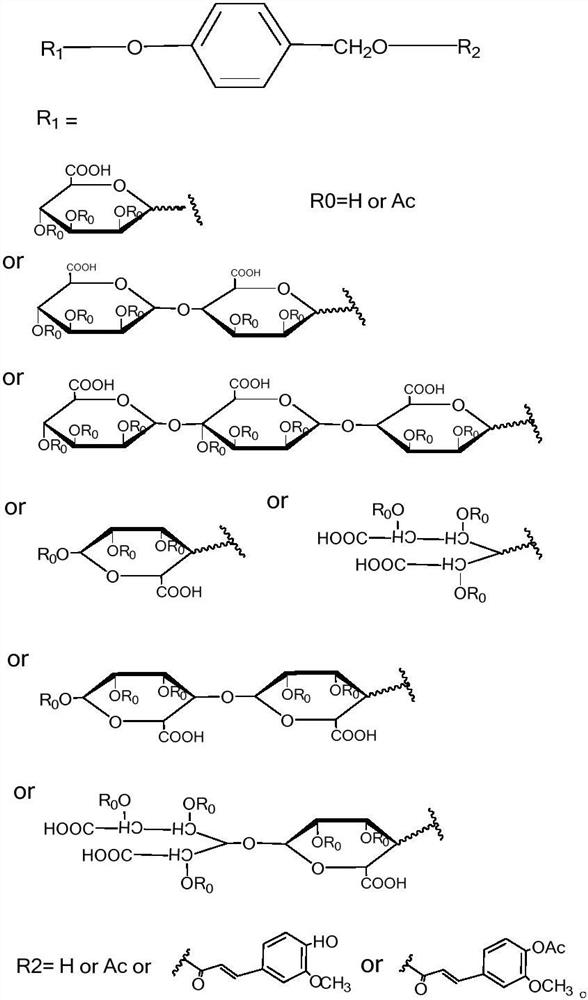
[0034] In the process of preparing the A series derivatives, it is first necessary to prepare 1-bromo-2,3,4-acetyl-6-formylmannide, and 3-{3-methoxy-4-[(2-acetyloxy Base) benzoyloxy] phenyl} acrylic acid, which can be prepared according to the preparation methods well known to those skilled in the art, and there is no special limitation in this application.
[0035] (1) Using p-hydroxybenzaldehyde as raw material, in anhydrous dichloromethane, add 1-bromo-2,3,4 acetyl-6-formyl mannoate and boron trifluoride ethyl ether successively under nitrogen protection, room temperature After the reaction is completed, add saturated sodium bicarbonate solution, separate the organic phase, wash with saturated sodium bicarbonate and water, dry over anhydrous sodium sulfate, and obtain p-acyl mannide benzaldehyde through column chromatography, p-acyl mannide benzaldehyde Under the catalysis of NaBH4, the aldehyde group can...
Embodiment 2
[0072] Example 2. Protective effect of gastrodin-derived compounds on nerve cell damage induced by Aβ aggregates
[0073] Using the cell viability without adding Aβ1-42 as a negative control, observe the inhibitory effect of a series of compounds on the neurotoxicity induced by Aβ. The specific implementation steps are as follows: Inoculate PCl2 cells in MEM complete culture medium and place them in a 96-well plate After culturing in a constant temperature cell incubator for 24 hours, add the pre-aggregated Aβ protein oligomers. After 2 hours, add a series of compound solutions to each well with a concentration of 200ug / ml, and continue to incubate in the incubator for 24 hours. After the end, the cell viability was measured by MTT method. Three parallels were performed each time, and the experiment was repeated three times.
[0074] The results in Table 1 show that the gastrodin derivatives of the present invention have a better protective effect on nerve cells, and have a s...
Embodiment 3
[0077] Embodiment 3, series compound A-I to CoCl 2 Protective Effects of Induced Hypoxic Injury on Nerve Cells and Cardiomyocytes
[0078] without CoCl 2 The cell viability of the induced nerve cells PC12 and cardiomyocytes H9C2 was used as a negative control to observe the effect of a series of compounds on the induction of CoCl 2 The inhibition of hypoxic injury of nerve cells and cardiomyocytes, the specific implementation steps are as follows: inoculate PC12 and H9C2 cells in MEM or DMEM complete culture medium, place them in a 96-well plate for culture, put them in a constant temperature cell incubator and incubate for 24 Hours later, the pre-dissolved injury solution containing CoCl2 was added, and after 2 hours, a series of compound solutions were added to each well with a concentration of 200ug / ml, and the incubator continued to incubate for 48 hours. After the end, the cell viability was measured by MTT method. Three parallels were performed each time, and the expe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com