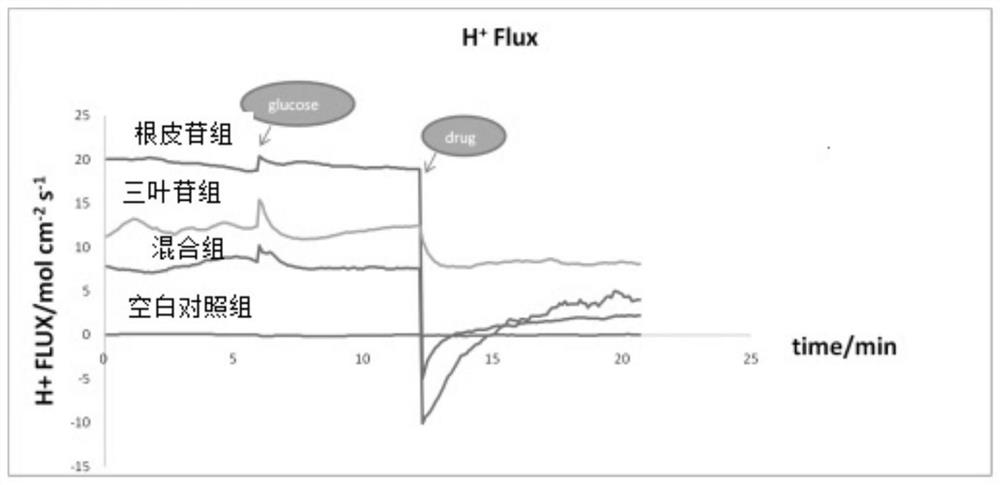
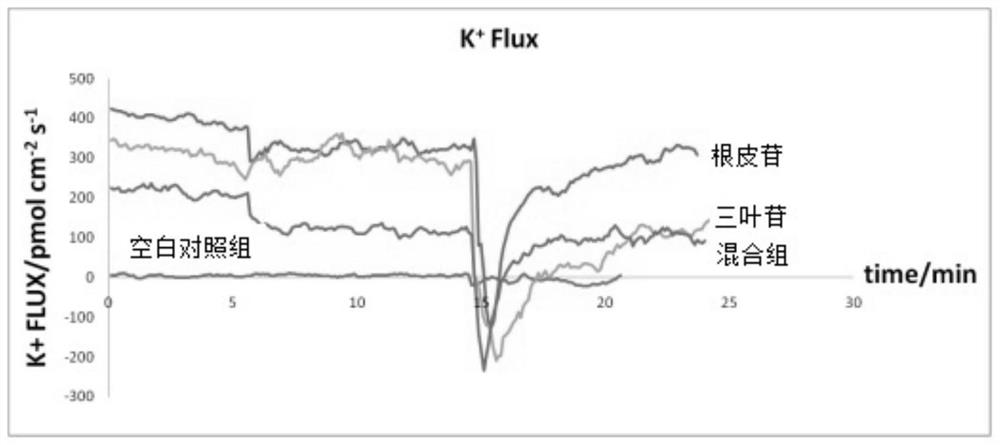
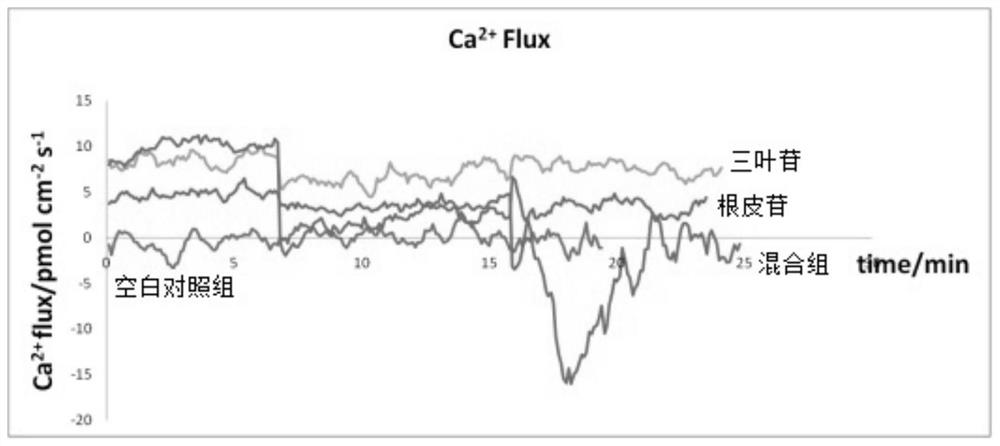
A kind of method for preparing phloridzin mixed hypoglycemic preparation
A phloridzin and hypoglycemic technology, applied in the directions of drug combination, pill delivery, pharmaceutical formulation, etc., can solve problems such as loss of activity, and achieve the effects of prolonging shelf life, preventing inactivation and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A method for preparing a mixed hypoglycemic preparation of phloridzin, comprising the steps of: microencapsulating phloridzin and trilobulin, and then adding xylitol, microcrystalline cellulose, maltodextrin and magnesium stearate as auxiliary materials Made into tablets or capsules. The mass ratio of phloridzin, trilobatin, xylitol, microcrystalline cellulose, maltodextrin and magnesium stearate is 0.1:0.15:1.5:0.3:2.7:0.03.
[0035] The microencapsulation treatment comprises the following steps:
[0036] (1) Pulverize phloridzin and trilobitin into fine powder, pass through a 100-mesh sieve, add 5 times of the fine powder with warm water at 60°C, and add 1% mannooligosaccharide and 1.5% sucrose fatty acid by mass of the fine powder. Glycerides were sonicated for 20 min to obtain a core material system;
[0037] (2) Mix the soybean lecithin, linseed gum and chitosan with a mass ratio of 6:2:1 evenly, then add the mixture to the core material system, stir evenly, and ...
Embodiment 2
[0040] A method for preparing a mixed hypoglycemic preparation of phloridzin, comprising the steps of: microencapsulating phloridzin and trilobulin, and then adding xylitol, microcrystalline cellulose, maltodextrin and magnesium stearate as auxiliary materials Made into tablets or capsules. The mass ratio of phloridzin, trilobatin, xylitol, microcrystalline cellulose, maltodextrin and magnesium stearate is 0.1:0.17:2:0.4:2.3:0.05.
[0041] The microencapsulation treatment comprises the following steps:
[0042](1) Pulverize phloridzin and trilobitin into fine powder, pass through a 100-mesh sieve, add 6 times of fine powder with warm water at 70°C, and add 0.9% mannooligosaccharide and 0.9% sucrose fatty acid by mass of fine powder Glycerides were sonicated for 30 min to obtain a core material system;
[0043] (2) Mix the soybean lecithin, linseed gum and chitosan with a mass ratio of 8:2:2 evenly, then add the mixture to the core material system, stir evenly, and the mass r...
Embodiment 3
[0046] A method for preparing a mixed hypoglycemic preparation of phloridzin, comprising the steps of: microencapsulating phloridzin and trilobulin, and then adding xylitol, microcrystalline cellulose, maltodextrin and magnesium stearate as auxiliary materials Made into tablets or capsules. The mass ratio of phloridzin, trilobatin, xylitol, microcrystalline cellulose, maltodextrin and magnesium stearate is 0.1:0.20:1.5:0.5:2.5:0.04.
[0047] The microencapsulation treatment comprises the following steps:
[0048] (1) Pulverize phloridzin and trilobitin into fine powder, pass through a 100-mesh sieve, add 7 times of the fine powder with warm water at 70°C, and add 0.5% mannooligosaccharide and 1.0% sucrose fatty acid by mass of the fine powder. Glycerides were sonicated for 20 min to obtain a core material system;
[0049] (2) Mix soybean lecithin, flaxseed gum and chitosan with a mass ratio of 6:3:1, then add the mixture to the core material system, stir evenly, and the mass...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com